هذه المحاضرة البسيطة تندرج تحت قسم (الكيمياء التحليلية) أو بالأخص بقسم (التحليل باستخدام الأجهزة) وهي مختلفة قليلا عن الكيمياء التحليلية مع أنهما يتبعان نفس المبادئ العلمية, وهي من المواد المهمة جدا في الأقسام الطبية لاسيما منها الصيدلانية, تعمل على دراسة الجوانب المختلفة مما يسمى (الكروماتوجراف) وهي آلية تحليل المادة الى مكوناتها الدقيقة للتعرف على محتوياتها, وهذه طريقة قديمة حديثة مهمة جدا ويعتمد عليها بشكل كبير جدا في مختبرات الأدوية والتشاخيص وتحديد السموم, وفي هذا الجزء من سلسلة المحاضرات نتناول دراسة سريعة حول تقنية حديثة نوعا ما من الكروماتوجراف, تستخدم فيها المضخات لضخ المادة المذيبة الى داخل السائل الذي يحتوي على المواد المراد قياس نسبها ونوعيتها, وتستخدم فيها الأجهزة المعملية الحديثة لهذا الموضوع والجميل في هذه التقنية انها استحدثت الأساليب القديمة وأضافت عليها التكنولوجيا الحديثة والكمبيوترات السريعة وباستخدام المعادلات الرياضية الطبيعية في علوم الجبر , تمكن الباحثون من استخلاص آلية جميلة تساعد على استخلاص المواد المكونة للمادة الواحدة, ومقارنتها وببعضها البعض ومقارنتها أيضا برسوم جبرية ثابتة القياس بوجود نفس الظروف, مما يؤدي الى سرعة في تحديد نوعية المواد وماهيتها بشكل دقيق
دراسة ومراجعة اسماعيل العبد مرتضى
يمكن تزويدكم بالمراجع المستخدمة عند الطلب ويرجى الضغط على الصور المنشورة لتكبيرها وقراءة محتوياتها وخصوصا عند الصور المتعلقة بالرسوم الجرافية الخاصة بتقييم المواد الناتجة عن عملية التفاعل الكاملة في النهاية
يمكن تزويدكم بالمراجع المستخدمة عند الطلب ويرجى الضغط على الصور المنشورة لتكبيرها وقراءة محتوياتها وخصوصا عند الصور المتعلقة بالرسوم الجرافية الخاصة بتقييم المواد الناتجة عن عملية التفاعل الكاملة في النهاية
High Performance liquid chromatography (HPLC) is a form of liquid chromatography which is used to separate compounds that are dissolved in a solution. It is a so much sophisticated form of column chromatography, In which fine particles are used and therefore require high pressure pumping systems to deliver the mobile liquid phase, the instruments of HPLC are as the following
A) Mobile phase reservoirs & solvent treatment system
B) Pumping systems
C) Columns & Packing
D) Detectors
B) Pumping systems
C) Columns & Packing
D) Detectors
A) Mobile phase reservoirs & solvent treatment system

Any modeRn HPLC is equipped with one or more solvent reservoirs, often there is a filter for removing dust & particulate matter from the solvents. It is also equipped with devices that introduce solvents for either isocratic elution or gradient elution (for more informations about those 2 elution procedures, please refer back to the first parts of this chain of lectures), They are designed to degas the mobile phase in use so as to
a) Eliminate dissolved gases, particularly oxygen, which may react with the mobile or stationary phases
b) To reduce the possibility of bubbles forming in the detector during the chromatographic process
b) To reduce the possibility of bubbles forming in the detector during the chromatographic process
B) Pumpming System
In HPLC, the resistance of the mobile phase the flow through the column is relatively high; thus high pressure is also required in order to perform this process, so pumping systems are used which can be divided into 2 major groups which are as the following
a) MECHANICAL PUMPS; which holds the advantage of having pulseless flow of solvent, constant flow rate, high pressure & usable in solvent programming devices
b) PNEUMATIC PUMPS; which are operating by compressed air, it’s advantages ranges between having lower costs, simple operation & pulse free
b) PNEUMATIC PUMPS; which are operating by compressed air, it’s advantages ranges between having lower costs, simple operation & pulse free
C) Columns & Packing
Columns used are stainless tubes usually 25cm in length & 4-6 mm in internal diameter. Guard Columns are sometimes used & are placed before analytical columns to protect them from extraneous material in samples that could cause changes in the performance of the analytical columns. Also they extend the lifetime of the analytical columns
The type of the material to be packed into the column is determined by the type of chromatography that will be used (LLC, LSC, Ion Exchange or Gel Filtration) & the type effects our choice for sure
D) Detectors
The most popular types of detectors in commercial instruments are as the following
a) Ultra Violet Detectors
b) Refractive Index Detectors
c) Heat of Absorption Detectors
b) Refractive Index Detectors
c) Heat of Absorption Detectors
A) Ultra Violet Detectors : These are the most widely used detectors. They can detect a few nanograms of a solute which have a moderate UV absorption. They can be very sensitive but also have obvious limitations to certain classes of compounds, To improve the detection power, the solutes can be treated with reagents after separation (post column derivatization). Clearly the solvent should not absorp UV light at the chosen wave length, & hence gradient elution may be used with suitable solvents w
المزيدHistory of Chromatography
مايو 12th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,History of Chromatography
This Article had been taken from the following book reference
The Text book of Pharmaceutical Analysis, Volume II
Instrumental Methods
Dr.A.V.KASTURE, Dr.S.G.WADODKAR, Dr.K.R.MAHADIK, Dr.H.N.MORE
Nirali Prakashan
Published in September, 2006
Instrumental Methods
Dr.A.V.KASTURE, Dr.S.G.WADODKAR, Dr.K.R.MAHADIK, Dr.H.N.MORE
Nirali Prakashan
Published in September, 2006
هذه الفقرة هي نبذة مأخوذة تشرح تاريخ إكتشاف وتطور آلية (الكروماتوجراف) التحليلية والتي أصبحت آلية مستخدمة على نطاق واسع ليس فقط في المجال الطبي ولكن أيضا في المجال البترولي والجيولوجي والبيولوجي وفي العديد من التطبيقات التحليلية الإكتشافية والصناعية في عالمنا المعاصر, أخذت هذه المعلومات من أحد المراجع الطبية المذكورة أعلاه وهي فقط للمهتمين بدراسة تاريخ وأسلوب تطور العلوم في بعض النواحي, معلومات عامة فقط للمهتمين بالإطلاع
نقل بواسطة اسماعيل المرتضى دون أي تغيير في المحتوى المنقول
History of Chromatography
The study of chromatography started in the 18′th century when with a great interest the nature of in-organic compounds was studied on filter paper by Runge. He separated in-organic salts & observed that inorganic salts travel to different extent producing an attractive pattern. In the year 1898, DAY in USA forced crude petroleum through a colmun of Limestone & Fuller’s Earth. He observed that first portion was of light hydrocarbons, followed by hydrocarbons of aromatic nature, un-saturated type, heterocyclic & nitrogen-sulphur containing high molecular weight hydrocarbons
The chromatographic principle was discovered first by a russian botanist, Michael Tswett (1906) who used a glass column of calcium carbonate for the separation of chlorophyll pigments from plants by using petroleum ether, The pigments according to their adsorption patterns, were resolved into various coloured zones, he then separated & estimated them
Between 1910 & 1930 very little work was published about chromatography, The major development occured around 1930 when Lederer & co-workers (1931) separated lutein & xanthine on a column of calcium carbonate powder, Further developments soon followed when Kuhn, Karrer & Ruzicka, separated plant carotenes into several components by adsorption chromatography. This helped in the resolution of naturally occuring mixtures of pigments, sugars, amino acids, proteins, vitamins & hormones. This leaded to the development of adsorption & partition column chromatography for the identification, separation, isolation, both on preparative & analytical cases
In 1935, Adams & Holmes observed some synthetic ion-exchange resins which are capable of exchanging ions & thus ion-exchange chromatography came into existence
Tiselius (1940) & Claesson
المزيدChromatography Part 6, Ion Exchange Chromatography, IEC
مايو 10th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography Part 6, Ion Exchange Chromatography, IEC
Clinical Pharmacy, Instrumental Analysis Section
A study in Instrumental Analysis as a revision for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
هذه المحاضرة البسيطة تندرج تحت قسم (الكيمياء التحليلية) أو بالأخص بقسم (التحليل باستخدام الأجهزة) وهي مختلفة قليلا عن الكيمياء التحليلية مع أنهما يتبعان نفس المبادئ العلمية, وهي من المواد المهمة جدا في الأقسام الطبية لاسيما منها الصيدلانية, تعمل على دراسة الجوانب المختلفة مما يسمى (الكروماتوجراف) وهي آلية تحليل المادة الى مكوناتها الدقيقة للتعرف على محتوياتها, وهذه طريقة قديمة حديثة مهمة جدا ويعتمد عليها بشكل كبير جدا في مختبرات الأدوية والتشاخيص وتحديد السموم, وفي هذا الجزء من سلسلة المحاضرات نتناول دراسة سريعة حول الكروماتجراف الأيوني, والذي يعمل على فصل الأيونات (مواد ذات شحنات كهربائية) مختلفة من مصدرها الجامع الأساسي, متعرفين بذلك على جرعتها ونسبتها ونوعية مكناتها في الخليط, أعتبر شخصيا أن هذه العلوم تعطي الانسان قدرة كبيرة على تمييز الأمور بشكل عام في مجال حياته العامة وليس فقط المجال الطبي, حيث انك تتعامل مع مجهول لتجعله معلوما, تقوم بدراسته وتتعب في تفسير مكنوناته لتحصل على نتيجة صحيحة ومرضية في النهاية
دراسة ومراجعة اسماعيل العبد المرتضى
يمكن تزويدكم بالمراجع المستخدمة عند الطلب
IEC is used for the separation of ionic substances (substances with charges) or their derivatives, which range from simple in-organic compounds & organic ions, to poly-electrolytes such as enzymes, proteins, hormones & nucleic acids
Ion Exchange Chromatography materials consists of polymers containing ionizable functional groups, They are water insoluble & the functional groups are usually simple, For example Sulphonic Acid in cation-exchange resins, Or Quaternary ammonium groups in anion-exchange resins, Thus there is an insoluble phase with fixed ionic sites of one charge
The mobile phase is an ionic material, such as acid, base or salt dissolved in water & so carefully chosen that it can compete with the sample ions for the ionic sites on the ion exchanger, The simple ion exchange mechanism is the most common one & can be shown in the following reaction
X-ve + R+Y- —–> Y-ve + R+X-ve ANION EXCHANGE
X+ + R-Y+ —–> Y+ + R-Y+ CATION EXCHNAGE
Where the following
X= Sample Ion
Y= Counter ion in the mobile phase, which have same charge as the sample
R= Ionic site on the ion exchanger, stationary phase
Y= Counter ion in the mobile phase, which have same charge as the sample
R= Ionic site on the ion exchanger, stationary phase
Note that Cation means positive charge & Anion means negative charge
As shown above, in anion exchange chromatography the sample ion X- is in competetion with the mobile phase counter ion Y- for the ionic sites on the ionic exchange resin R+, Similarly, in cation exchange chromatography, the sample cation X+ is in competetion with the mobile phase counter ion Y+, for the ionic sites R-, on the ion exchanger
Simple ion exchange separations are based on the different strength of the solute ion\resin (ion pair) interactions, Solutes interacting weakly with the resin in the presence of the mobile phase counter ions are poorly retained on the column & elute early in the chromatogram (lower K’ value), Solutes that interact strongly with the resin (stationary phase) are more strongly retained & elute later, with higher K value’
Ionic complexes of neutral molecules can sometimes be used for ion exchange separations, A sugar in the presence of borate ions forms a sugar\borate anion
Sugar + BO= —–> Sugar.BO
Thus complex mixtures of various neutral sugars have been separated in this manner in anion exchange resins
Note that K’ value is the amount of a solute (sample) retained in the column, which mainly depends on the strenght of the interaction between the solute & the stationary phase
TYPES OF ION EXCHANGE MATERIALS
The materials differ in the nature of the exchange groups, which is incorporated into each & in the micro-structures that each has, they are classified as the following
A) Ion Exchange Resins; which includes both the Cationic Exchangers & the Anionic Exchangers
B) Ion Echange Gels
C) Ion Exchange Cellulose
B) Ion Echange Gels
C) Ion Exchange Cellulose
A) Ion Exchange Resins
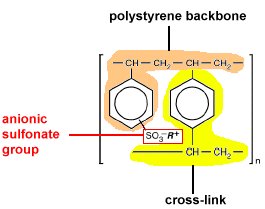
They have a very complex structure & are considered to be the most commonly used stationary phases, A typical resin is prepared by polymerization of styrene & divinyl benzene, The degree of cross linkages is determined by the ratio of divinyl benzene to styrene, Increasing the cross linking increases the rigidity, reduces swelling, reduces porosity & reduces the solubility of the polymeric structures, the ion exchange resins are either cationic or anionic exchangers
A) Cationic Exchanger
Which contains Anionic Resins (-ve phase), The Acidic functional groups are easily introduced for example by sulphonation in which a sulphonic acid group attached to nearly every aromatic nucleus, Sulphonic Acids are strong acids with essentially completely dissociated protons, although these protons are not free to leave the resin unless replaced by other positive ions
The total number of of equivalents of replacable protons per unit volume of resin determines the exchange capacity of the resin, so the exchange capacity depends on the number of the functional groups
Instead of sulphonic acid groups, carboxylate group can be added to the aromatic rings, weak acid groups may be introduced but the exchange distribution function depends on the strength of the acid
B) Anionic Exchangers
Which contains cationic resins (+ve phase), The basic groups are introduced; the resin can exchange anions rather than cations. Strong anion exchangers are prepared with tertiary amine yielding a strongly basic quaternary ammonium group, Weak anionic exchangers can be prepared with secondary amines
Which contains cationic resins (+ve phase), The basic groups are introduced; the resin can exchange anions rather than cations. Strong anion exchangers are prepared with tertiary amine yielding a strongly basic quaternary ammonium group, Weak anionic exchangers can be prepared with secondary amines
B) ION EXCHANGE GELS
Sephadex ion exchange gels (sephadex which is a cross linked dextran) with an appropriate exchange group can be used for the separation of poly-electrolytes (this includes weakly basic, weakly acidic, strongly acidic & strongly basic) groups
C) ION EXCHANGE CELLULOSE
They are derived from carbohydrate polymers, A new improved fibrous products & micro-granular products have been introduced
SELECTIVITY OF RESINS
In chromatographic separations, the selection depends upon many factors (factors affecting the resolution power) such as the following
A) Size of the particles, This affects the rate of exchange & permeability of the packed column, For large-scale operation a fairly high flow rate through the resin bed is desirable & that requires the use of fairly large particles
B) Degree of cross-linkages, it influences the rigidity, porosity & swelling properties of the exchangers
C) Nature of the functional group or as we can say the nature of the ion exchanger
D) Strength of the functional group
E) Number of the functional groups, it determines the capacity of the resin
B) Degree of cross-linkages, it influences the rigidity, porosity & swelling properties of the exchangers
C) Nature of the functional group or as we can say the nature of the ion exchanger
D) Strength of the functional group
E) Number of the functional groups, it determines the capacity of the resin
The effect of the PH on the capacity of ion exchangers
المزيدChromatography Part 5, Gel Chromatography
أبريل 14th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography Part 5, Gel Chromatography
Clinical Pharmacy, Instrumental Analysis section
A study in Instrumental Analysis dedicated for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
It’s called also Gel permeation, Molecular sieving or Molecular Exclusion, It is a particular type of liquid-liquid chromatography (partition chromatography) used for the separation of substances according to the differences in sizes of their molecules, or as called according to the difference in the Relative Size of the molecule
There are different materials used in gel filtration which are as the following
A) SEPHADEX
It is a cross-linked dextran (polysaccharaid polymer), which at the macroscopical level the product is in the form of spherical beads, because of its high content of hydroxyl groups, it have great affinity for water, therefore, it will swell up in water or electrolyte solutions to give semi-transparent gel particles
It is a cross-linked dextran (polysaccharaid polymer), which at the macroscopical level the product is in the form of spherical beads, because of its high content of hydroxyl groups, it have great affinity for water, therefore, it will swell up in water or electrolyte solutions to give semi-transparent gel particles
B) Bio-Gel Series
It is suitable for gel filtration in aqueous media, It is based on cross linked poly-acrylamide gels
It is suitable for gel filtration in aqueous media, It is based on cross linked poly-acrylamide gels
C) Agarose
It is a neutral polymer derived from agar, It is used for the fractionation of substances of really high molecular weights as certain polysaccharaids, proteins & nucleic acids
It is a neutral polymer derived from agar, It is used for the fractionation of substances of really high molecular weights as certain polysaccharaids, proteins & nucleic acids
D) Specially Modified Media
It is a product derived from the dextran-based gels by reacting the hydroxyl groups with a reagent to render them hydrophobic, The modified gel particles swell in non-aqueous solvents
It is a product derived from the dextran-based gels by reacting the hydroxyl groups with a reagent to render them hydrophobic, The modified gel particles swell in non-aqueous solvents
A new gel, sephadex LH-20 has recently became available, some of the hydroxyl groups of the dextran gel are alkylated so that the gel will swell in polar organic solvents, water or mixtures of the 2
Styro-gel, it is a rigid cross-linked polysterene gel
THE PRINCIPLE OF SEPARATION
The fractionation range of these gels depends upon the pore size & this in turn is inversely proportional to the amount of cross-linking agent used, The greater the amount of cross-linking agent used so the less the swelling properties of the gel
The usuall way of characterizing various types of gel is by means of their water regain value (WRV), This represents the amount of water (in ml) that is retained by 1g of the dry gel grains, The type numbers of sephadex & the bio-gel series are TEN times the WRV, sephadex G10 has a WRV of 1 & sephadex G2000 has a WRV of 20, These values doesn’t include the water between the grains
The types with low WRV have smaller pore size & are used for the fractionation of small particle molecules, while types with high molecular weight are fractionated with high water regain gels, Thus sephadex G10 will fractionate substances with molecular weight up to 700 & sephadex G2000 will fractionate globular proteins & peptides with molecular weights ranging from 5000 up to several hundered thousands, other types cover intermediate ranges
SEPARATION TECHNIQUES
المزيدChromatography Part 4, Column Chromatography
أبريل 7th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography Part 4, Column Chromatography
Clinical Pharmacy, Instrumental Analysis section
A study in Instrumental Analysis dedicated for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Column chromatography forms a closed system that all types of chromatography may be carried out, partition, adsorption, ion exchange or even gel chromatography, because you can fill it’s column with any type of stationary phase, remember that the stationary phase means the stable phase & the mobile phase means the moving phase
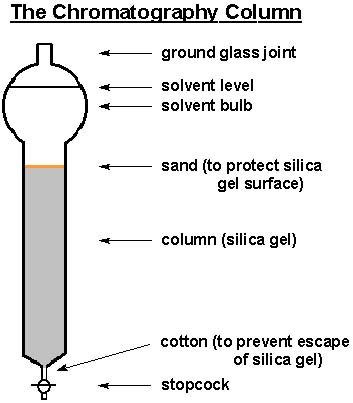
A column is usually a glass tube with constant cross sectional area & a tap at the bottom, Various accessories are attached for maintenance of the elution process & for the collection of separated substances such as in the below picture, accessories like sand & cotton …. etc
A column consists of 2 phases that are classified in the following manner
Solid & Liquid phases, this is an adsorption or ion exchange method
Two Liquid Phases, This provides the partition system, including gel chromatography
A gas & liquid or gas & solid, The gas providing the mobile phase & this is in GLC & GSC, Respectively
COLUMN PACKING
In each form the stationary phase must be packed in the column tube in such a manner that the chromatographic process proceeds effeciently, & the column size will be choosed depending on the type & quantity of the sample & solvent….etc needed
The size of the solid particles is important for 2 reasons, firstly, & especially when using an adsorbent, an adequate surface area must be exposed, so the particle size of the adsorption phase plays an important role in determining the surface area, smaller particle sizes provides higher surface area, but dividing the particle size must not be too small also not to affect the elution process, 2′nd, the eluting liquid must be near optimum speed. In partition process the solvent velocity must be low enough to allow equilibrium to be achieved
The column must be perfectly vertical in position & a small cotton piece should be placed at the tap opening to avoid the loss of any of the column ingredients
APPLICATION OF THE SAMPLE
The mixture is dissolved in a suitable solvent to give a fairly concentrated solution that is introduced into the packed column by a pipette, Care should be taken so that the surface of the supporting solid is not disturbed, Another method of application is to use small discs of filter paper of a size just to fit on the top of the column, The mixture is adsorbed on the discs & then placed on the column after drying, A third method is to mix the mixture with some of the supporting solid until a dry powder is obtained, The powder is then put on top of the column, what is really important is to avoid disturbing the surface of the supporting solid in anyway, disturbing the surface will just affect the whole process in a negative way directly + the application of the sample must be always on the TOP of the column
ADSORPTION COLUMNS
Many solids have been used as adsorbents & the most popular adsorbents are silica gel & alumina (as mentioned in TLC in last article also), The adsorbents used are granular heavy powders, which are easy to be packed into a column, The particle size of the adsorbent should be small enough to minimize diffusion effect & ensure that the equilibrium is rapidly attained, on the other hand, The particles should not be too small to impede the flow of the solvent as just mentioned in an above paragraph of this article, very small powder particles will hinder the movement of the solvent & will affect the chromatography process in a negative way
PARTITION COLUMNS
The stationary liquid is supported in a solid media that is inert (as in all past mentioned chromatography methods) it must be inert to the substances which are supposed to be separated, The supports most commonly used are silica gel, cellulose powder & Kieselghur, Starch has found more limited use
The support material must expose as mush high of surface area as it can to the mobile phase, It must be mechanically stable, easy to pack into the column & it must not impede the solvent flow, Packing the column & application of sample are exactly similar to those used on adsorption columns
THE SEPARATION TECHNIQUE
The column is operated by elution analysis (isocratic or gradient elution), Frontal analysis or displacement analysis,The last 2 are not used commonly nowadays, The nature & solubility of the mixture influence the choice of the solvent, organic solvents are normally chosen depending on their elution power, for a sample of un-known nature or non-polar compounds, start with a very weak solvent in general, For gradient stepwise elution, the eluotropic (or eluototropic) series should be followed, In this series the solvents are arranged in order of their elution powers, The most common solvents are arranged as the following
Water > Methanol > Ethanol > Propanol > Acetone > Ethyl Acetate > Diethyl Ether > Chloroform > Dichloromethane > Benzene > Carbon Tetrachloride > Cyclohexane > Hexane > Petroleum Ether
المزيدChromatography Part 3, Thin Layer Chromatography TLC
أبريل 2nd, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography Part 3, Thin Layer Chromatography TLC
Clinical Pharmacy, Instrumental Analysis section
A study in Instrumental Analysis dedicated for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
In past articles I spoke about general instrumental analysis methods including chromatography in general, paper chromatography & now it’s the time to speak about Thin Layer Chromatography called TLC
TLC relies upon the production of a uniform layer of stationary phase held on a glass plate or other supporting medium, The most popular media used are microcrystalline cellulose & Kieselguhr, the last had been defined before in another article but microcrystalline cellulose in brief is a natural polymer which is widely used in pharmaceutical manufacturing processes for different reasons, It’s a purified, partially depolymerized cellulose prepared by treating alpha-cellulose, obtained as a pulp from fibrous plant material, with mineral acids. The degree of polymerization is typically less than 400. Not more than 10% of the material has a particle size of less than 5 m m like the pictures below in general
The past mentioned stationary phases holders are normally used in partition chromatography where a liquid is help as a stationary phase as mentioned before, in case the chromatography method was wanted to be adsorption method so Alumina is used normally with silica gel, the last had been used for both partition & adsorption methods
The solid layers are prepared by applying a slurry of the chosen medium in a suitable solvent (water is the most commonly used solvent) on a clean glass plates, if the material adhere badly, a binding agent such as CaSO4 is often incorporated in small quantities, the slurry is spread on the plate by mean of commercial spreading appartus, this appartus is used to help producing a uniform spreaded layer & to control the thickness of the layer depending on the experiment or process itself, the thickness of the layers might varie between 0.25 - 0.5 up to 1 mm
TYPES OF STATIONARY PHASES
There is a wide range of adsorbents commercially available such as the following
Silica gel , silicic acid; a partition method basically but can be used for both types
Alumina, aluminium oxide; an adsorption method
Kieselguhr or diatomaceous earth; a partition method
Microcrystalline cellulose; a partition method
Other commercial adsorbents
A) SILICA GEL OR SILICIC ACID
Is the most commonly used type & is slightly acidic in nature, & used in both partition & adsorption methods, a binding agent is normally incorporated to improve it’s binding properties to the glass plate, an agent such as Calcium Sulphate Hemihydrate, this can creat problems in TLC of inorganic compounds, but it had been found that adsorbents will adhere quite satisfactory in the absence of the binder if they are ground to a smaller particle sizes (I relate it to the magnetic power or exactly charges achieved or something similar to that normally), 2 UV indicators had also been incorporated, either in a single form or together, these are Sodium fluorescein & Sodium salt of hydroxy pyrene sulphonic acids (they fluoresce at 254 nm & 365 nm, respectively) thus providing a contrasting background for compounds which adsorb at this frequency, in the separation of organic bases (like alkaloids) which are basic in nature usually a small amount of ammonia or diethylamine is added to the solvent system to enhance the dissolving properties because the CaSO4 is acidic in nature also
B) ALUMINA OR ALUMINIUM OXIDE
It is a slightly basic adsorbent (used basically in adsorption methods) with less resolution compared to silica, it is also more chemically reactive adsorbent so that it is possible for sensitive compounds to be degraded while the plate is being loaded that’s why you need to be careful while using it with sensitive compounds, sometimes in the separation of acids a small amount of glacial acetic acid is added to the solvent system to enhance the resolution properties, it is available with or without plaster of CaSO4 & fluorescin indicator
C) Kieselguhr or diatomaceous earth
It is a neutral adsorbent (used basically in partition methods) having a lower resolving power, it can be mixed with silica gel usually to give a less active mixed adsorbent & to increase it’s adsorption capacity , but it is mainly used as a support for the stationary phase during partition TLC , it is available normally with or without a binder
D) Microcrystaalline cellulose
It is used only as a support for the stationary liquid phase in partition chromatography TLC, the advantages of cellulose TLC over PC (Paper Chromaatography) is that running times are greatly reduced compared with those for the same solvent system on paper, & sensitivity is greatly increased, this is explained partially by the reduced fibre length in cellulose for TLC & partialy by the higher specific surface area, both of which tends to make the spots more compact & because of it’s fibrous nature, cellulose gives satisfactory layers without a binder needed in the method
E) Other commercial adsorbents
Those especially used in TLC include the following
Polyamides, used basically in TLC for flavonoids & other organic compounds
Magnesium silicate
PLATE COATING
Plates should be cleaned with a detergent solution first, then rinsed with hot water or with an organic solvent to free the surface from grease, this oil or grease will prevent a professional adherance of the future layers which are supposed to be spreaded on the glass plate & finally dried before using it. The appropriate adsorbent is a slurry made with distilled water; the proportion of adsorbent to water varies with the type of the adsorbent (for example for silica gel it is 1:2), after that to mix the slurry throughly it is preferable to stir it mechanically, for cellulose plates high speed stirring with a blender is advisable. The usual thickness of layers of TLC is 0.25 nm (for analytical purposes), & thicker layers (0.5 to 1 mm) are used for preparative purposes. Normal chromatoplates are prepared in standard sizes 5 X 20, 10 X 20 & 20 X 20 cm, as shown in the pictures using a glass plate with the stationary phase slurry covering it by manual ways in the lab
Activation & storage of the plates
Plates for adsorption chromatography are normally left for about 30 minutes after spreading, then placed in a metal racks & kept in an oven for at 105 degree C. for 30-60 minutes to drive off the water content but using higher temperatures will lead to the destruction of the paper itself or the plate, Then the activated plates should be stored in a desiccator until being used, The activity of Alumina plates is governed to a greater extent by the temperature to which are heated & the storage after activation is important because any subjection to humidity will lead to adsorb & absorb water in the plate which will lead to the in-activation of the plate again
LAYER IMPREGNATION
It is used for partition chromatography basically, water or an aqueous solution can be used as a stationary phase & in this case the coated plates (with silica gel, cellulose or Keiselghur) are allowed to dry at room temperature and heated for 10 minutes, In some cases hydrophylic or hydrophobic liquids (such as in reverse methods discussed in past articles) are used as a stationary phase, impregnation is performed as in PC in that case & the layers are allowed to dry without heating
Precoated plates are available commercially with a wide range of coating materials to make it easier for the chromatographer
SAMPLE PREPARATION & APPLICATION
It is the same as in PC (Paper Chromatography) but unlike PC in sample application, the drawing of the base line should be avoided since it scraps the coated layer, so several plastic templates are available to protect the coated layer surface during the loading process, For preparatice TLC mechanical applicators are used, so as shwon in the picture down this is a WRONG WAY OF DRAWING THE LINE unlike PC method
PLATES DEVELOPMENT
Development of TLC is normally done by the Ascending methods (explained in PC before), It should be carried in a saturated tank, After development, the solvent front is marked & the plates are dried rapidly in a stream of warm air
المزيدChromatography Part 2, Paper Chromatography PC
مارس 24th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography Part 2, Paper Chromatography PC
Clinical Pharmacy, Instrumental Analysis section
A study in Instrumental Analysis dedicated for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Paper chromatography is a typical partition system where the stationary phase is water held on cellulose molecules which in turn are kept in a fixed position by the fibrous structure of the paper, this method is used normally to separate the mixtures containing similar chemical nature components
The advantages of the PC possessed for separating mixtures os small size, high resolution, ease of detection & simplicity of appartus, so it have high separation capacity with high level of accuracy
TYPES OF PAPER
A variety of specially prepared grades of paper is available, as a general rule the following can be used
Whatman No.1 (Schleicher), used for analytical work
Schull 2043, used for analytical work
Whatman No.3, used for analytical work
Whatman No.3 MM is normally made thicker & can be used for preparative work
Above is a sample picture of whatman filter papers, Whatman chromatography papers are the most widely used papers for chromatography worldwide. This acceptance and usage reflect the purity, high quality, and consistency of Whatman papers. These qualities are relied upon by chromatographers and are essential to successful, reproducible chromatography. Whatman chromatography paper media are made from specially selected cotton cellulose. They are rigorously quality controlled for characteristics important to the chromatographer and to ensure uniformity within the grade
SOME CHROMATOGRAPHIC DEFINITIONS
Development : It is the process of allowing the solvent to move along the filter paper, which means allowing the mobile phase (solvent) to pass among the stationary phase
Retardation Factors (Rf Values) : Which is the ratio of the distance in which the substance moves, compared with the distance reached by the solvent front, both measured from the point of application of the sample
The equation of the retardation factor maybe as the following
Rf= Distance moved by the substance (sample) devided by The Distance moved by the solvent (front of the mobile phase) ; this is an accurate definition
The Rf value is influenced by a number of factors including the temperature, the composition of the solvent & the time of equilibrium before actual development, Note that if the atmosphere was not saturated then evaporation will occur from the edges of the filter paper & deviation will happens, & that the longer is the distance moved by the solvent so the better is the separation process, Rf values are used for the identification purposes, as identical substances have indentical Rf values normally when chromatographed under the same conditions, Rx is the ration between the distance moved by a substance to the distance moved by a standard substance
Resolution : Is the degree of separation of compounds after development in chromatographic methods
Loading: Is the amount (quantity) of the mixture (sample) applied as a spot or streak to the paper
How to choose the material & Solvent systems
The 2 factors that have the greatest effect on the separations for any particular mixture are the solvent & the treatment of the paper as the following
Aqueous Stationary Phase : In which water in this type is held as an stationary phase on a paper and a solvent which is immiscible with water is used as a mobile phase, this solvent is preferred to be cheap, pure, non-toxic, low volatility & naturally dissolve the substance under the test
Stationary hydrophylic solvents: In some cases polar hydrophilic solvents may be used as an stationary phase like methanol & formamide, the paper is either saturated with the vapours of these solvents (if they are volatile) or dipped in a dish containing the solvent in a volatile organic solvent (formamide in acetone) followed by drying, by the way formamide is non-volatile thats why we use acetone as a solvent for formamide, then we dip the paper in them then after removing the saturated paper, acetone will evaporate leaving the original formamide phase in the chromatographic paper
Stationary Hydrophobic solvents (Reversed Phase): In this type a hydrophobic solvent is held as stationary phase & the mobile phase is hydrophylic, in this sytem the sheet of paper may be impregnated with the hydrophobic substance (for example parrafin), the hydrophylic character of the paper may be modified chemically, usually by esterification & then the modified paper will then take the hydrophobic solvent vapours
For example in general you can take the chromatographic filter paper & dip it in olive oil or parrafin so in this way the paper will be saturated with a hydrophobic phase or by chemical treatment apply to the cellulose paper (which is polar with high hydroxyl groups) apply an acetyl group so treat it with acetylation or esterification by this way you can change the polarity of the chromatographic paper easily
APPLICATION OF SAMPLES
Samples are to be dissolved in a suitable volatile solvent, a pencil line is drawn parallel to one edge of the paper & at a suitable distance from it, then a drop of the test solution is spotted onto the appropriate position by a capillary tube or platinium loop, If more test solution is required the first spot is allowed to dry & a similar application is repeated, but I prefer to start it & do it once directly, better for the accuracy of the process, the process is shown in the picture above under the title, as you see a sample is spotted & a line by pencil is drawn then the paper is just touching the solvent below the line & enough time is taken for the solvent to ascend till it reach the sample spotted and keep on moving holding with it some of the mixture components which dissolved in the solvent & forming a solvent front at the end
المزيدChromatography part 1 Introduction
مارس 23rd, 2009 كتبها اسماعيل العبد مرتضى نشر في , Chromatography studies, Instrumental Analysis studies,Chromatography part 1 Introduction
Clinical pharmacy, Instrumental analysis section
A study in Instrumental analysis dedicated for professionals & students
Done By Ismail Mortada
B.Sc.Pharamcy & Health Sciences, Clincal Pharmacy section
KROMATOS= Colour;GRAPHOS= Written; those are the greek origins of the word, which means writting by colours
Introduction
The direct definition of chromatography is a technique by which complex mixtures whatever organic or inorganic can be resolved into individual components & we use it normally for compounds identification & analysis, it’s the separation or isolation of a mixture into it’s pure individual components OR we can say that it’s the distribution of different individual pure components of a mixture between 2 immiscible phases one of them is stable (holds the compound) & one is mobile or moving phase
Chromatographic separations are achieved by the distribution of components in a mixture between a fixed & a moving phase respectively, separations begin to occur when one component is held more strongly by the stationary phase than the other, stationary phase means the stable phase or the un-moving or non-mobile phase, so one compound will be held stronger in this phase compared to the other one which tends to move fatser in the mobile phase, all this depends on the chemical structure & nature of the mixture components depending also on bonding and interactions between the different phases & the components themselves
Chromatographic methods are classified depending on the following
On the nature of the mobile phase as a first choice classification, this phase might be liquid or gas in nature
On the nature of the stationary phase which might be solid or liquid but NOT GAS in nature
Now depending on the nature of the stationary phase chromatography is classified to the following
If the stationary phase is solid so it’s called Adsorption Chromatography
If the stationary phase is liquid so its called Partition Chromatography
Depending on that; we have 4 major subdivisions & types of chromatography which are as the following
Adsorption with liquid mobile phase called LSC= Liquid Solid Chromatography
Adsorption with Gaseous mobile phase called GSC= Gas Solid Chromatography
Partition with liquid mobile phase called LLC= Liquid Liquid Chromatography
Partition with Gaseous mobile phase called GLC= Gas Liquid Chromatography
Note: In the naming system the first name is given for the mobile phase nature & the 2′nd name is for the stationary phase
ESSENTIAL FEATURES OF CHROMATOGRAPHIC SEPARATION ARE
The Competition of the 2 phases, the mobile & the stationary; with each other to contain the mixture components
The components will be distributed in the 2 phases according to their distribution coeffecient or also called Partition coeffecient (K) Which have a special equation in which K= Amount of solute/unit stationary phase devided by Amount of solute/unit mobile phase
The Distribution coeffecient depends on many factors including solubility, volatility, adsorption properties, molecular size or ionic charge in ionizable molecules
The mobile phase flows past the stationary phase which will allows the separation process to succeed
In simple words we can say that chromatography depends on having a compound (mixture of 2 or more organic or inorganic components) which we need to analyse & understand it’s nature, so we bring a kind of 2 phases one is stable and one is moving and we keep the compound on the stable phase & then we move or pass the mobile phase on it, this will lead to an interaction between all the collection of compounds & the phases leading to the separation of the components (pure) of the compound by time depending on different factors; some of which were mentioned above, & depending on the nature of the 2 phases 5 types of general chromatography had been known till now which were introduced because of the introduction of new stationary phases which are as the following
Partition Chromatography which includes a liquid film coated in an inert suitable support
Adsorption Chromatography which includes finely divided solid functioning as an adsorbing surface & they are divided finely to increase their surface area
Ion Exchange Chromatography (which is reversible step) which includes ionic groups (ionic means holding different charges) which are attached to an inert material; this method is used in purifying water for example & the competition will be between the sample (water considered mobile phase also) & the stationary phase directtly
Gel Chromatography (also called molecular sieving/Gel filteration/Gel permiation/Molecular exclusion) which depends on a suspension of a polymer having a suitable pore size (like agar) & is an important method for some analysis kinds such as separating hormones, enzymes & biological fluids; AGAR itself is a polymer with pores, so small particles will enter into the pores & might leave only in case it dound a larger pore to enter in it
Zone Electrophoresis which depends on a suitable support moistened with a buffer solution & it is not a typical chromatography because it lacks a mobile phase but we can use it with other chromatography methods to increase & improve the separation function & results purity
BASIC TECHNIQUES OF CHROMATOGRAPHY
There are different techniques of how to apply different chromatography types in which it includes the following
Paper Chromatography PC, which is primarily a partition process; it’s the most simple method in which we use a simple filter paper in the process such in the picture below
Column Chromatography CC; might be partition, adsorption ion exchange or Molecular exclusion; which means gel chromoatography; in this method we bring an innert column & we fill it with the stationary phase, then we apply the mobile phase & we leave it to pass through natural gravity, & the tape down of the column will control the solvent flow, in this method we can separate large quantities
Thin Layer Chromatography TLC; might be partition, adsorption ion exchange or Molecular exclusion; which means gel; in this method normaly we bring a stationary phase and we prepare a slurry (paste) form then we apply the lsurry to inert support by using special devices because the thickness of the layer should be the same through all the sides of the sample, the thickness will be about 0.25 to 1 mm, then after coating it we have to dry it, anyways we’ll speak more about it in future articles
Gas Chromatography GC, It might be a partition process GLC or an adsorption process GSC
High Performance Liquid Chromatography HPLC, might be partition, adsorption ion exchange or Molecular exclusion; which means gel
CHOICE OF THE METHOD
You need to choose the good method to have a true results, it is usual to try simpler methods or techniques such as PC or TLC first because they can provide a useful guide to the type of the system to be used later on, Generally the choice depends on the substances which are to be separated as the following
For substances of similar chemical types, partiton chromatography is normaly used
For substances of different chemical types, Adsorption chromatography is normaly used
For gases & volatile substances, Partition chromatography is used
For ionic & inorganic substances, Ion Exchange & Zone Electrophoresis chromatograaphy is used
To separate ionic from non-ionic substances, Ion Exchange & Gel Chromatography is used
For biological materials & compounds of high molecular weight, Gel Chromatography or Electrophoresis is used
ADSORPTION CHROMATOGRAPHY
In this type, the stationary phase is solid & the mobile phase is gas or usually liquid, For good separation, the component of the mixture should have different degree of affinity for the adsorbent & the interaction between adsorbent & component should be reversible; because if it wasn’t we will not have the ability to separate and measure the sample later on & we’ll loose one of the components. The separation occurs when one component of the mixture is more strongly adsorbed than the others by the stationary phase
The components of the mixture is bound to the solid surface by specific interaction forces such as the following
Ionic or electrostatic forces
Dipole-Dipole interactions
Dipole Induced Dipole interactions
Simple London Forces
The separation is dependant on the following also
The surface area of the adsorbent, so the adsorbent should be in a fine particle size (finaly divided solid particles) by which decreasing the particle size, will lead to increasing the surface area, so the functionality of the stationary phase
The temperature & the concentration of the sample detected, so at a given temperature the relation ship between the amount of the substance on the stationary phase (Cs) and the amount in the mobile phase (Cm) is expressed as Adsorption Isotherm, so the adsorption isotherm is the distribution of the solute between 2 phases within a given temperature
The nature & affinity of the phases & the compound itself
Types of adsorption Isotherm
Three types of adsorption isotherms are observed normally which are the linear, convex & concave
When the line goes near the V axes that means there is more affinity toward the stationary phase & vice versa toward the mobile phase
Each point in the above image will have a special location depending on it’s concentration & adsorption rate & it’s affinity to one of the phases
Points and lines will cause one of 2 shapes which we call them Tailing or Fronting, imagine a dot of color on a piece of tissue, it it moves downwards it will cause fronting & it it moves upward then it will cause tailing…etc
PARTITION CHROMATOGRAPHY
In this type the stationary phase is a liquid, frequently water, held on a suitable inert porous supporting solid such as cellulose. The mobile phase can be a gas or liquid mixture, More than one solvent is always present in the liquid mobile phase, separation occurs between 2 components of a mixture when one component is more strongly retained than the other by the stationary phase.
In this method as just mentioned we can use 2 or more solvents as a mobile phase & the compound which will be more retained or strongly retained will move in a slow motion & by changing the strength of the solvent we can change the speed
GENERAL ADVANTAGES OF CHROMATOGRAPHIC METHODS
It is possible to achieve separations which are very difficult by other methods like the separation of isomers
Decomposition of the substances being separated is less than in other methods, this is an important consideration in cases of labile substances which are oftenly of biological origins
Only a very small quantity of the mixture is required, indeed; it is not possible to handle very large quantities by chromatographic methods and this might constitue a disadvantage for preparative purposes
Chromatographic techniques are simple & rapid & the appartus required is cheap
At Gas chromatography we use high temperature because we need to separate gases & volatile substances
Enzymes are thermolabile & can’t be separated by applying heat































ليست هناك تعليقات:
إرسال تعليق