B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
It’s called also Gel permeation, Molecular sieving or Molecular Exclusion, It is a particular type of liquid-liquid chromatography (partition chromatography) used for the separation of substances according to the differences in sizes of their molecules, or as called according to the difference in the Relative Size of the molecule
There are different materials used in gel filtration which are as the following
A) SEPHADEX
It is a cross-linked dextran (polysaccharaid polymer), which at the macroscopical level the product is in the form of spherical beads, because of its high content of hydroxyl groups, it have great affinity for water, therefore, it will swell up in water or electrolyte solutions to give semi-transparent gel particles
It is a cross-linked dextran (polysaccharaid polymer), which at the macroscopical level the product is in the form of spherical beads, because of its high content of hydroxyl groups, it have great affinity for water, therefore, it will swell up in water or electrolyte solutions to give semi-transparent gel particles
B) Bio-Gel Series
It is suitable for gel filtration in aqueous media, It is based on cross linked poly-acrylamide gels
It is suitable for gel filtration in aqueous media, It is based on cross linked poly-acrylamide gels
C) Agarose
It is a neutral polymer derived from agar, It is used for the fractionation of substances of really high molecular weights as certain polysaccharaids, proteins & nucleic acids
It is a neutral polymer derived from agar, It is used for the fractionation of substances of really high molecular weights as certain polysaccharaids, proteins & nucleic acids
D) Specially Modified Media
It is a product derived from the dextran-based gels by reacting the hydroxyl groups with a reagent to render them hydrophobic, The modified gel particles swell in non-aqueous solvents
It is a product derived from the dextran-based gels by reacting the hydroxyl groups with a reagent to render them hydrophobic, The modified gel particles swell in non-aqueous solvents
A new gel, sephadex LH-20 has recently became available, some of the hydroxyl groups of the dextran gel are alkylated so that the gel will swell in polar organic solvents, water or mixtures of the 2
Styro-gel, it is a rigid cross-linked polysterene gel
THE PRINCIPLE OF SEPARATION
The fractionation range of these gels depends upon the pore size & this in turn is inversely proportional to the amount of cross-linking agent used, The greater the amount of cross-linking agent used so the less the swelling properties of the gel
The usuall way of characterizing various types of gel is by means of their water regain value (WRV), This represents the amount of water (in ml) that is retained by 1g of the dry gel grains, The type numbers of sephadex & the bio-gel series are TEN times the WRV, sephadex G10 has a WRV of 1 & sephadex G2000 has a WRV of 20, These values doesn’t include the water between the grains
The types with low WRV have smaller pore size & are used for the fractionation of small particle molecules, while types with high molecular weight are fractionated with high water regain gels, Thus sephadex G10 will fractionate substances with molecular weight up to 700 & sephadex G2000 will fractionate globular proteins & peptides with molecular weights ranging from 5000 up to several hundered thousands, other types cover intermediate ranges
SEPARATION TECHNIQUES
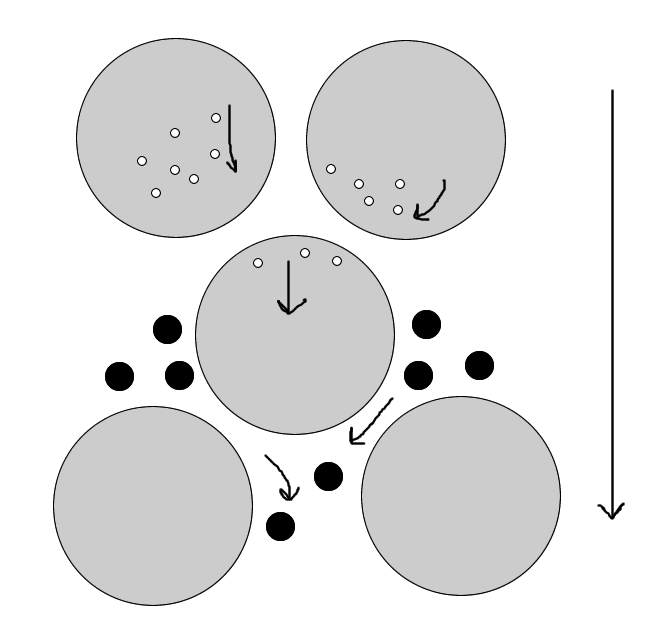
A) The sample is applied to the top of the column & then washed through the bed of the gel particles with water or buffered solution
B) substances with molecules larger than the largest pores of the swollen beads (above the exclusion limit) are not able to penetrate the gel particles & therefore pass through the bed in the liquid phase outside the particles & emerge from the column first
C) Smaller molecules penetrate the particles to varying extents depending upon their shape & size, There is thus a partition of the molecules between the liquid inside the gel particles & that outside, The smaller the molecules, the larger the percentage of liquid within the particles that is available to them
D) Molecules therefore leave the column in the order of decreasing molecular size, the larger size will leave the comlumn first followed by the smaller sizes depending on their partition (shape & size) ranges
Note the following
A) The flow of the mobile phase will cause larger molecules to pass through the column un-hindered, without penetrating the gel matrix, whereas smaller molecules will be retarded according to their penetration of the gel
B) The components of the mixture thus emerge from the column in order of relative molecular mass, the largest first, Any components that are completely excluded from the gel will not be separated from each other, and similarly, small molecules that completely penetrate the gel will not be separated from each other, Molecules of intermediate size will be retarded to a degree dependent on their penetration of the matrix
APPLICATION OF GEL CHROMATOGRAPHY
A) Analysis of mixtures of molecules of different molecular weight
B) Molecular weight determination, Although gel filtration depends on molecular size, investigations have shown that elution volumes of globular proteins on the sephadex G100 & G200 types are largely determined by their molecular weights. Over a considerable range, the elution volume is approximately a linear function of the logarith of the molecular weight, If a calibration curve of proteins of known molecular weight can be drawn up, the molecular weight of an unknown protein can be determined, This kind of work is very valuable in enzyme work
C) De-salting, one of the common separation problems is the removal salts & small molecules from macromolecules, The larger differences in distribution coeffecient makes it posssible to use simple column with high flow rate to de-salt mixtures or compounds
Chromatography Part 5, Gel Chromatography
Clinical Pharmacy, Instrumental Analysis section









ليست هناك تعليقات:
إرسال تعليق