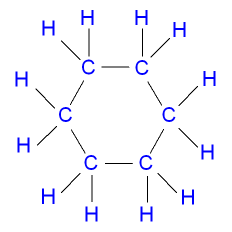
As I read on the BBC news like the others, they could do it right now, in which they could take a LIVE picture for a chemical molecule, What is really amazing is that it is very clear & identical to the usual chemical structures that we draw for organic compounds, this gives a great indication that science became so developed in a way that we can in the future become a real part of the whole universe chemical structures & that scientist will play a basic & direct role in SHAPING LIFE
المزيدALKANES, part 2, Preparation of Alkanes
يوليو 11th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Pharmaceutical Organic Chemistry, alkanes,Pharmaceutical Organic Chemistry, Saturated Hydrocarbons
ALKANES, part 2, Preparation of Alkanes
Clinical Pharmacy, Pharmaceutical Organic Chemistry Section
A study in Pharmaceutical Organic Chemistry as a revision for professionals & students
Studied & Revised By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Under the process of Masters in Business Adminstiration, Pharmaceutical Marketing
Under the process of Masters in Business Adminstiration, Pharmaceutical Marketing
هذه المقالة تعنى باستكمال مراجعة ودراسة أول طائفة من طوائف المواد الكيميائية العضوية (الموجودة والمكونة للكائنات الحية) وهي من المواد الشيقة والمهمة جدا في التطبيقات الطبية, البيولوجية, الأحيائية والصيدلانية, وبشكل مخصص وبعد أن تحدثنا في مقالات سابقة عن ماهية وتعريف المركبات العضوية, وبدأنا بشرح أبسط أنواعها او أقلها تعقيدا مما يسمى بالألكانات, نعنى الآن بطريقة تحضير هذه المواد (الألكانات) كيميائيا وكيفية تركيبها….هي دراسة سريعة ولكن أساسية…ودمتم سالمين..أخوك إسماعيل مرتضى
In last lecture we spoke about saturated hydrocarbons, basicaly the alkanes & we spoke about them, their nomencalture & different properties….etc, Now in this section of this topic we’ll discuss more about the preparation of alkanes
And although most alkanes upto pentane are obtained normaly from petroleum by fractional distillation, but still more complex alkanes can be prepared
Alkanes preparation can be achieved within different models & mechanisms, all have their own characteristics & ways including th following
A) Catalytic Hydrogenation of Alkenes
B) Hydrolysis of a Grignard Reagent
C) The Wurtz Reaction, which is called Dimerization
B) Hydrolysis of a Grignard Reagent
C) The Wurtz Reaction, which is called Dimerization
A) Catalytic Hydrogenation of Alkenes
This is a kind of what is called Additional reactions depends on the following points in which the carbon-carbon double bond in alkenes can add a mole of hydrogen in the presence of a suitable catalyst to give an alkane, in other words, adding hydrogen atom to a double bonded alkene structure in the presence of a catalyst will give us an alkane
The general mechanism includes the dissolving of an alkene in a suitable solvent in the presence of the catalyst, then hydrogen gas is passed (bubbled) at low pressure in the reaction vessel with constant stirring
Catalysts used can be such as the following
A) Platinum Pt
B) Nickel Ni
C) Palladium Pd
A) Platinum Pt
B) Nickel Ni
C) Palladium Pd
Example 1) For example the conversion of Ethylene to Ethane as the following
CH2=CH2 + H2 —-> CH3-CH3 In the presence of Pt & Pressure
In which the following
CH2=CH2 is Ethylene
CH3-CHE is Ethane
CH2=CH2 is Ethylene
CH3-CHE is Ethane
Example 2) Another example is the formation of n-hexane from 1-Hexene
CH3-CH2-CH2-CH2-CH=CH2 + H2 —-> CH3-CH2-CH2-CH2-CH2-CH3 In the presence of Ni & Presuure
In which the following
CH3-CH2-CH2-CH2-CH=CH2 is 1-Hexene
CH3-CH2-CH2-CH2-CH2-CH3 is n-Hexane
CH3-CH2-CH2-CH2-CH=CH2 is 1-Hexene
CH3-CH2-CH2-CH2-CH2-CH3 is n-Hexane
B) Hydrolysis of a Grignard Reagent
A) Grignard reagent is an alkyl magnesium halide compound, R-Mg-X
B) The Grignard reagent is formed when a solution of an Alkyl Halide (R-X) is allowed to stand over a metallic magnesium in the presence of anhydrous diethyl ether, a strong reaction will occur, the solution will turn cloudy & start to boil, The magnesium metal disappears & the resulting alkyl magnesium halide is called Grignard Reagent, this reaction is so active in a way that special precautions must be taken while preparing it
C) Than the addition of water to the grignard reagent will form alkane
B) The Grignard reagent is formed when a solution of an Alkyl Halide (R-X) is allowed to stand over a metallic magnesium in the presence of anhydrous diethyl ether, a strong reaction will occur, the solution will turn cloudy & start to boil, The magnesium metal disappears & the resulting alkyl magnesium halide is called Grignard Reagent, this reaction is so active in a way that special precautions must be taken while preparing it
C) Than the addition of water to the grignard reagent will form alkane
Precautions taken when preparing Grignard Reagent are as the following
A) There must be no water present in the reaction system
B) There must be no other functional groups available in which the Griganrd Reagent may react with, such as aldehydes, ketones, alcohols, amines & carboxylic acids, the presence of any of these groups destroys the reagent before it has the chance to produce the alkane or the needed product
المزيدB) There must be no other functional groups available in which the Griganrd Reagent may react with, such as aldehydes, ketones, alcohols, amines & carboxylic acids, the presence of any of these groups destroys the reagent before it has the chance to produce the alkane or the needed product
Saturated Hydrocarbons, ALKANES part 1
أبريل 14th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Pharmaceutical Organic Chemistry,Pharmaceutical Organic Chemistry, Saturated Hydrocarbons
ALKANES, part 1
Clinical Pharmacy, Pharmaceutical Organic Chemistry Section
A study in Pharmaceutical Organic Chemistry as a revision for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Hydrocarbons are organic compounds that contain only 2 elements which are Carbon & Hydrogen atoms only & they are normally devided into 2 classes which are as the following
A) Aliphatic Hydrocarbons divided also into 3 subclasses which are Alkanes, Alkenes & Alkynes
B) Aromatic Hydrocarbons which are compounds containing Benzene structures
B) Aromatic Hydrocarbons which are compounds containing Benzene structures
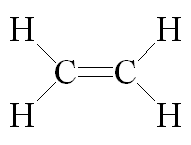
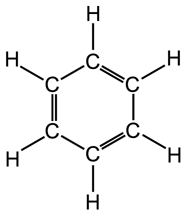
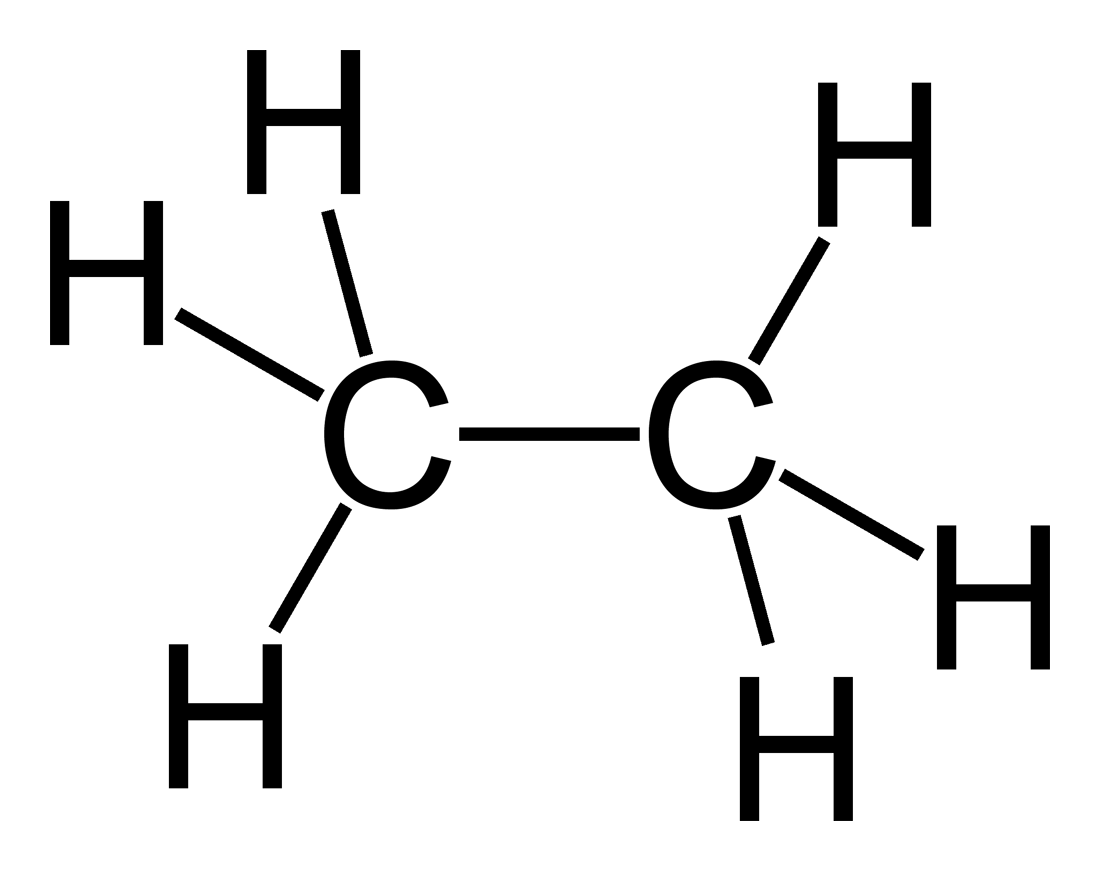
Note that the alkanes are also called Saturated Hydrocarbons because they are stable regarding chemical reactivity but Alkenes & Alkynes are called Un-Saturated Hydrocarbons because they contain double & triple bonds which are active from chemical point of view, this will be explained in more details in the future articles, the following pictures will show general differences in basic structure between ETHANE (alkane), ETHENE (alkene) & ETHYNE (alkyne) regarding the bonds characteristics
structure of ethane an alkane
Structure of ethene an alkene
Structure of ethyne an alkyne
Structure of benzene ring in aromatic hydrocarbons
ALKANES
Alkanes are characterized by carbon-carbon single bond, that’s why they are reffered to as Saturated Hydrocarbons as just mentioned before, they show a general lack of chemical reactivity, therefore, they are also called Paraffin hydrocarbons, they are extremely flammable, they constitute the fuels for heating gases in our homes
STRUCTURE OF ALKANES
sp3 Hybridization
For a normal carbon atom in which z=6 the electronic configuration would be as the following please revise the article before this one in the same section
The first energy level K= 1s2
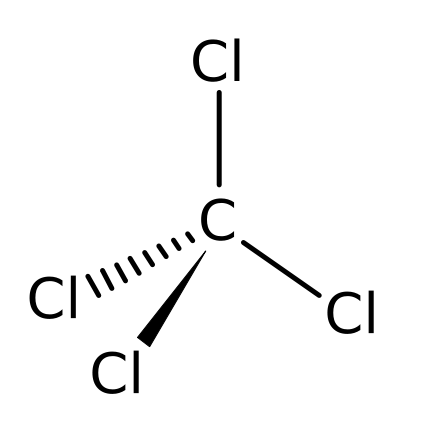
The 2′nd energy level L= 2s2, 2Px1, 2Py1 in this way we spent the 6 electrons 2 filled the first 1s orbital, 2 filled the 2′nd 2s orbital in L level & 2 left, one in the 2Px & 1 in the 2Py & the 2Pz will stay empty, this is the logical way of chemical distribution of the electrons (revise the first article on organic chemistry) & this means that carbon is suppose to do 2 bonds only (which means to be divalent) because it have only 2 un-shared free electrons, but in fact, carbon atoms showed to have 4 single bonds and to be tetravalent instead of being divalent with 2 single bonds only, for example as seen in ethane structure just mentioned upstairs, we see that a single carbon atome forms 4 single bonds or in the following structure of Carbon Tetrachloride, in which it formed 4 single bonds with 4 chloride atoms
The 2′nd energy level L= 2s2, 2Px1, 2Py1 in this way we spent the 6 electrons 2 filled the first 1s orbital, 2 filled the 2′nd 2s orbital in L level & 2 left, one in the 2Px & 1 in the 2Py & the 2Pz will stay empty, this is the logical way of chemical distribution of the electrons (revise the first article on organic chemistry) & this means that carbon is suppose to do 2 bonds only (which means to be divalent) because it have only 2 un-shared free electrons, but in fact, carbon atoms showed to have 4 single bonds and to be tetravalent instead of being divalent with 2 single bonds only, for example as seen in ethane structure just mentioned upstairs, we see that a single carbon atome forms 4 single bonds or in the following structure of Carbon Tetrachloride, in which it formed 4 single bonds with 4 chloride atoms
So to explain this, the theory of sp3 Hybridization was discovered in which by providing energy to the carbon atom, one electron from the ground state of 2s2 orbital will promote & be activated to leave the orbital & go to fill the empty 2Pz orbital in the L level state
So carbon will leave from the ground state to become in the activated state & then the 2s orbital will mix (hybridize) with the rest 2P orbitals to form 4 hybridized orbitals , for sure they have to hybridize to have 4 equal bonds, the process is shown in the following picture, note that if they didn’t hybridize this means that 4 single bonds will occur, 3 of them are equivalent (3 from the p orbitals) and one will be not equivalent to them because it merge from a lower energy level
So Hybridization is the merging of an s orbital & 3 p orbitals to give 4 equivalent sp3 orbitals, this hybridization will be by head-head of the orbitals forming what is known as sigma bond & giving the carbon a tetrahedron structure like that of methane the simplest organic compound in nature which is composed of only one carbon atom only
The structure of the first member in organic compounds & alkanes which is the simplest form composed of one carbon atom only & called methane & is not available purely directly in nature
Note that the new energy hybridization of the orbitals formed a new orbital called sp3 orbital which an energy which is higher then the 1s orbital & lower then the 2p orbitals
ETHANE
This is the 2′nd member of the alkane series, it’s composed of 2 carbon atoms so it’s molecule is larger then methane by -CH2- only & note that the difference between each member & the other in alkanes would be the same -CH2 molecule so we can say that methane is CH4 but ethane is C2H6 or CH3-CH3
PROPANE
It is the 3′rd member so it have one more carbone atom -CH2 compared to ethane & the structure would be C3H8 or CH3-CH2-CH3, as just mentioned, the fact is that every member of the alkanes series differs from the next member by a methylene group which is -CH2, the alkanes series is a homologous series
ISOMERIC STRUCTURE OF ALKANES
Isomers are different compounds with identical molecular formulas, they are readily distinguishable from each other because they exhibit differences in their physical & chemical properties, so they exhibit different chemical reactions
The first 3 members of the alkane series, the methane, ethane & propane have NO ISOMERS because there is only one way in arranging the molecule in 3D structures but the rest of the alkanes series might have an isomer, which is called structure isomers, see the following example
By comparing the structures of butane (the 4′th member in the series) & iso-butane we see that they have different molecular structure although they have the same number of carbon & hydrogen atoms, both have the same chemical formula which is C4H10 but they still differ in their properties & they are considered 2 different compounds (structural isomers) the first having a boiling point of zero degree C & the 2′nd having a boiling point of -12 degree C also from the past boiling points we can see that the straight chain are more stable than the isomer form, note that straight chains naming starts by the letter n, so we say n-butane & not butane alone to differentiate it from iso-butane
THE NOMENCALTURE OF ALKANES
The alkanes general formula is CnH(2n+2) which is stable for all the alkanes members, where n= the # of carbon atoms in the molecular structure
There are 2 basic systems for the naming of alkanes which are the following
A) Common Names , also called general or primary names or the un-systematic name
B) The IUPAC Name, or the systematic name
B) The IUPAC Name, or the systematic name
A)The Common Names of Alkanes
These are as the following
Holding 1 carbon, named METHANE, with a molecular formula of CH4 & Structural formula of CH4
Holding 2 carbons, named ETHANE, with a molecular formula of C2H6 & Structural formula of CH3-CH3
Holding 3 carbons, named PROPANE, with a molecular formula of C3H8 & Structural formula of CH3-CH2-CH3
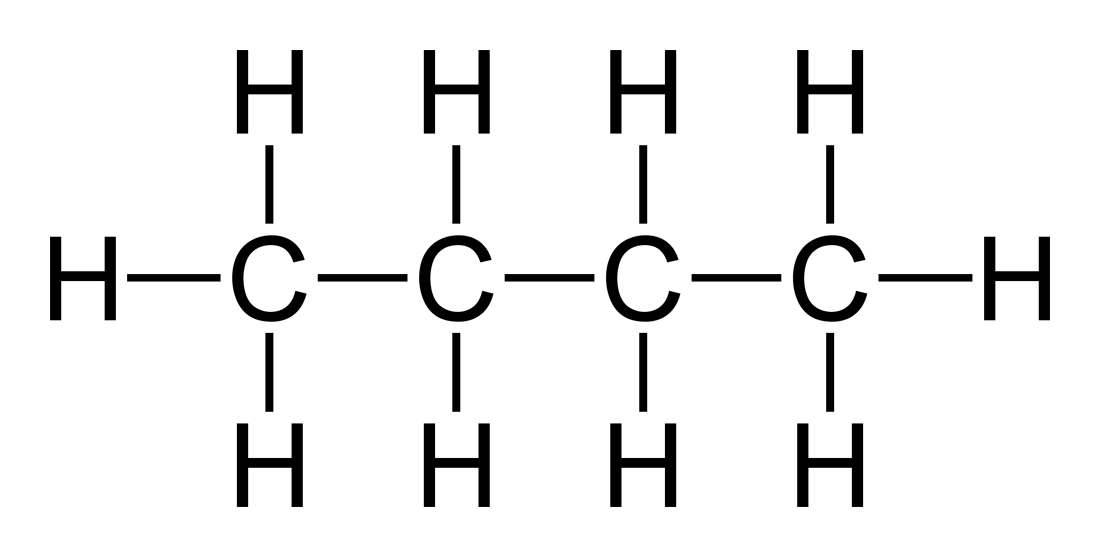
Holding 4 carbons, named BUTANE, with a molecular formula of C4H10 & Structural formula of CH3-CH2-CH2-CH3
Holding 5 carbons, named PENTANE, with a molecular formula of C5H12 & Structural formula of CH3-CH2-CH2-CH2-CH3
Holding 6 carbons, named HEXANE, with a molecular formula of C6H14 & Structural formula of CH3-(CH2)4-CH3
Holding 7 carbons, named HEPTANE, with a molecular formula of C7H14 & Structural formula of CH3-(CH2)5-CH3
Holding 8 carbons, named OCTANE
المزيدHolding 2 carbons, named ETHANE, with a molecular formula of C2H6 & Structural formula of CH3-CH3
Holding 3 carbons, named PROPANE, with a molecular formula of C3H8 & Structural formula of CH3-CH2-CH3
Holding 4 carbons, named BUTANE, with a molecular formula of C4H10 & Structural formula of CH3-CH2-CH2-CH3
Holding 5 carbons, named PENTANE, with a molecular formula of C5H12 & Structural formula of CH3-CH2-CH2-CH2-CH3
Holding 6 carbons, named HEXANE, with a molecular formula of C6H14 & Structural formula of CH3-(CH2)4-CH3
Holding 7 carbons, named HEPTANE, with a molecular formula of C7H14 & Structural formula of CH3-(CH2)5-CH3
Holding 8 carbons, named OCTANE
Pharmaceutical Organic Chemistry, Introduction
أبريل 10th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Pharmaceutical Organic Chemistry,Pharmaceutical Organic Chemistry, Introduction
Clinical Pharmacy, Pharmaceutical Organic Chemistry Section
A study in Pharmaceutical Organic Chemistry as a revision for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Organic chemistry is one of the major sciences in general chemistry studies & one of the most important chemical sciences in pharmaceutical & medical studies, because organic chemistry deals directly with the medication nature & structure including our bodies also nature & structure, it is defined simply as the study of any compound containing a carbon atom inside it
picture of carbon atom artificially
It is called organic because scientists used to think that these compounds were found only in living things or fossils, & there are about 5 million compunds, 4.5 million of them are organic in nature, in addition to that, wide number of other carbon-containing compounds can be now produced artificially in the laboratories, note that we can produce organic compounds (containing carbon) from in-organic compounds also like in the synthesis of urea, as seen in the picture below, urea is made from Ammonium cyanate (in-organic) by Heat application
Synthesis of urea under wohler 1828 reaction discovery
The unique properties of carbon among other atoms
Carbon ranks only twelfth (12th) in the abundance among the elements
Carbon consists of less than 0.1% of the earth’s crust, oceans & atmosphere
Carbon consists of less than 0.1% of the earth’s crust, oceans & atmosphere
Before discussing the structure of carbon atom I would like to speak just in general about chemical bonding idea, there are some facts in chemistry which indicates that all the atoms in nature would tend to interact with anything (eachother) to reach the noble gases configuration systems, those noble gases & normally stable because they possess a complete balanace, most of those noble gases have (8 electrons) in their balance except helim having only 2 electrons, so all the elements will interact to achieve an outerside balanced octect structure as to become as similar as to the nearest noble gas element in the periodic table, a picture or periodic table is seen downstairs
So to reach the balance state, basically 2 ways are done which includes Ionic Bonding reactions or Covalent bonding reactions, the first is a reaction in which a transfer of electrons will occur , but , the 2′nd is a reaction in which sharing of electrons will occur, both of them tend to re-balance the outer shells to reach the balanced stage
the reaction between chloride & sodium forming sodium chloride as an example of ionic bonds
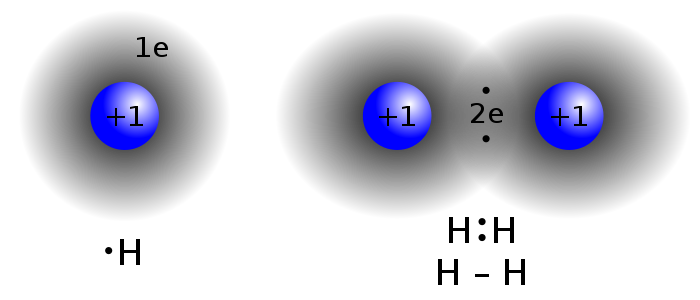
The formation of hydrogen molecule as an example of covalent bonding
Elements on opposite sides on the periodic table tend to have ionic bonding, & those who have nearer locations on the periodic table tend to have covalent bonding, There is also some thing called the polar-covalen bonds, in which the electrons shared between 2 atoms are not staying in equal spaces between the 2 nucleus of the atoms, I mean the shared electrons will be more near to one of the 2 atoms (this happens only with 2 different atoms) anyways, this will provide the 2 atoms with partial sharges and it will be called sigma bonds
STRUCTURE OF CARBON ATOM
Carbon in periodic table is in group IVA
Carbon atom is tetravalent, which means it can form 4 single bonds
Carbon must form 4 bonds to satisfy its Octect of electrons
Bonds maybe with other carbons or with other elements
Carbon atom is tetravalent, which means it can form 4 single bonds
Carbon must form 4 bonds to satisfy its Octect of electrons
Bonds maybe with other carbons or with other elements
TYPES OF CARBON COMPOUNDS
Tetravalent C atom, like in a single carbon atom
Straight chain arrangement, like in Butane
Branched chains, Like in Iso-butane
Ring arrangement, like in cyclo-hexane
Combination of carbons + other elements other than hydrogen like in 2,chloro-butane
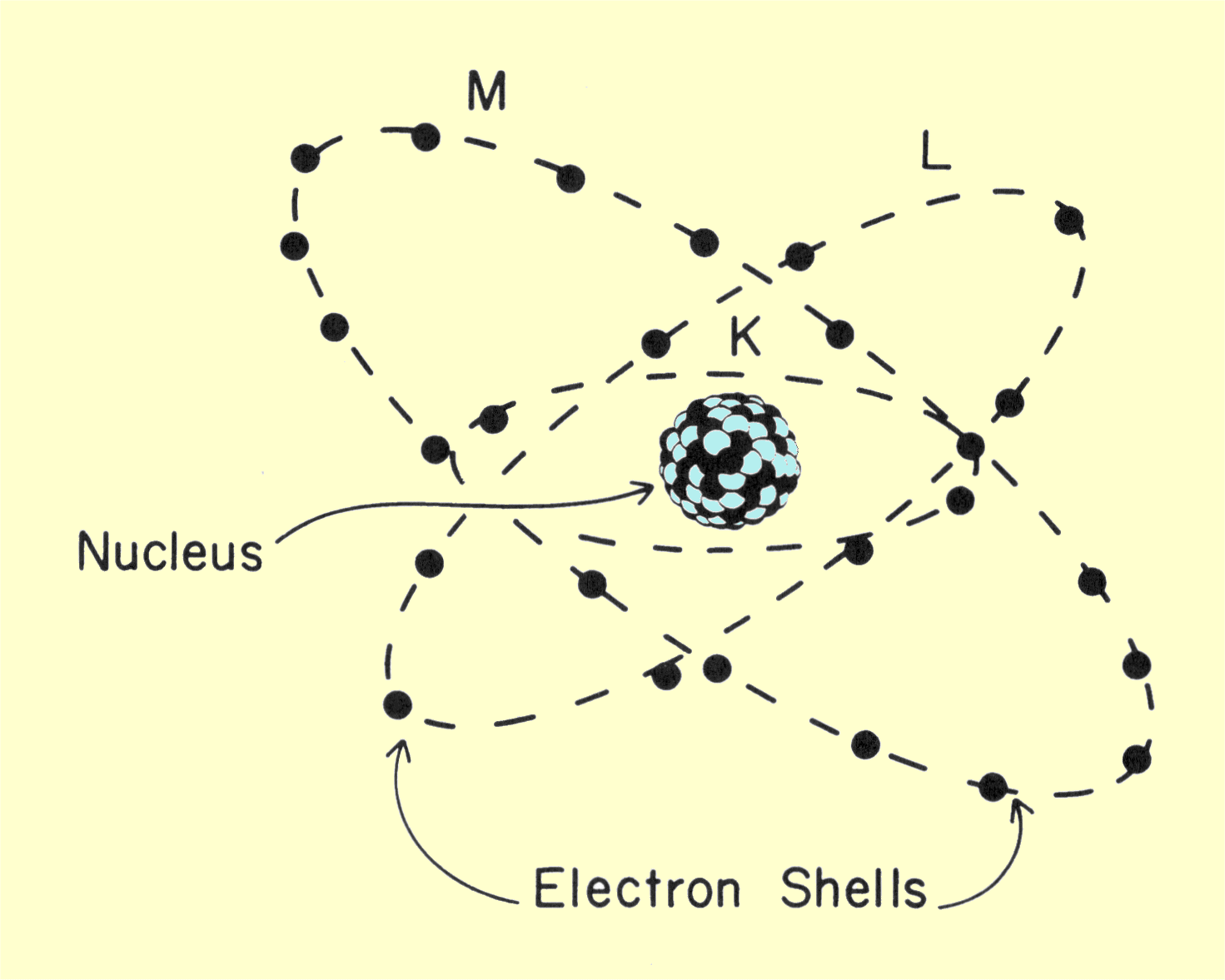
The structure of the atom
Atoms consists of positive nuclei surrounded by negative charged electrons all are inside the atomic orbita media
In a normal neutral atoms the sum of the negative charges (electrons) equals to the number of positive charges in the nucleus (protons) or as called the atomic number, in addition the nucleus contains the neutrons also
Electrons are distributed around the nucleus in successive shells (energy levels) of increasing radius, those shells are lighter than the nucleus so that’s why they surround it
Each energy level is assigned for identification with a letter and\or a number, which is called a Principal Quantum Number (n) which is very important, this number is given to each shell or each energy level, the electrons occupying the orbitals are also increased in the energy whenever they are getting more far from the nucleus core, because whenever they get more far so the nucleus can’t attract them anymore, so they have higher energy to leave & interact with other atoms or elements
The first shell (K) holding the number 1
The 2′nd shell (L) holding the number 2
The 3′rd shell (M) holding the number 3
The 4′th shell (N) holding the number 4
The 5′th shell (O) holding the number 5
The 6′th shell (P) holding the number 6
The 7′th shell (Q) holding the number 7
The 2′nd shell (L) holding the number 2
The 3′rd shell (M) holding the number 3
The 4′th shell (N) holding the number 4
The 5′th shell (O) holding the number 5
The 6′th shell (P) holding the number 6
The 7′th shell (Q) holding the number 7
While going from (K) to (Q) the energy level will increase, as mentioned I mean the energy level of the electrons
The maximum number of electrons possible to occupy a shell is equal to 2n(2) for example
The shell K in which n=1 so max. # of electrons to occupy this shell equals 2*1(2)=2 electrons
The shell M in which n=3 so max. # of electrons to occupy the shell equals 2*3(2)= 2*9= 18 electrons
The shell M in which n=3 so max. # of electrons to occupy the shell equals 2*3(2)= 2*9= 18 electrons
Within each shell, the electrons are further grouped into one or more orbitals, each orbital will hold 2 electrons in opposite spins or sides
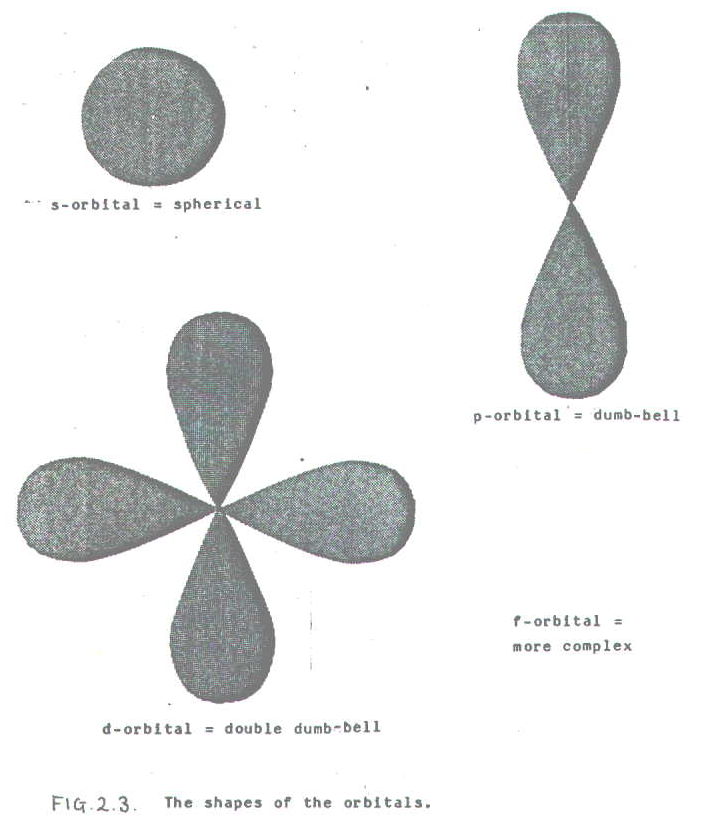
Atomic orbitals are designated by the letters s,p,d & f & each atomic orbital have a special shape as shown in the picture below
The number of orbitals in a given shell equals the square of the principal quantum number n n(2) as for the following example
For the shell K in which n=1 the # of orbitals equal 1(2)= 1 orbital
For the shell M in which n=3 the # of orbitals equal 3(2)=9 orbitals
For the shell M in which n=3 the # of orbitals equal 3(2)=9 orbitals
ORBITAL SHAPES
s orbitals are spherical shaped electron clouds, it contains only one basic shape as shown in the following pictures
p orbitals are dunbbell-shaped electron clouds, they have different orientations (3 shapes) called Px, Py & Pz as shown in the following pictures
FILLING THE ORBITALS
Electrons occupy orbitals of lower energy content before those of higher energy content for example an electron will occupy the 1s orbital before the 2s orbital, & the 2s orbital before the 3 equivalent 2p orbitals and so on
THE ELECTRONIC CONFIGURATION
PAULI’S EXCLUSION PRINCIPLE
It stated that only 2 electrons can occupy any atomic orbital , and to do so these 2 must have opposite spins, which means opposite directions, this means the following
There can be a maximum of only 2 electrons in the 1s orbital
There can be a maximum of only 2 electrons in the 2s orbital
There can be a maximum of only 2 electrons in each of the 2p orbitals
There can be a maximum of only 2 electrons in the 2s orbital
There can be a maximum of only 2 electrons in each of the 2p orbitals
For An Example
notations, the electron distribution of Neon where Z=10
So 1s2 , 2s2 , 2px2, 2py2, 2pz2 or 2s2 , 2p6
ELECTRONIC CONFIGURATION: is the electron distribution of an atom in its various orbitals, those electrons are arranged (as mentioned before) in energy levels
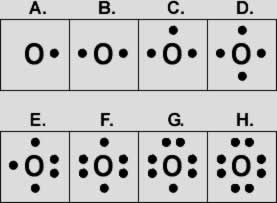
THE LEWIS ELECTRON GOT DIAGRAM
In most chemical reactions, only the outer shell (valence) electrons are involved, The lewis electron dot diagram is used to represent the valence electrons basically, as the following example of the electronic configuration of oxygen in which the atomic number is 8 so the configuration would be 1s2 , 2s2, 2px2, 2py1, 2pz1
now in the electron distribution, 2 electrons must fill each orbital so the 1s orbital would be filled easily then we’ll have 6 electrons left, 2 electrons will fill the 2s orbital, then we’ll have 4 electrons left, now there must be an electron filling each orbital first then we’ll still have one electron left so this will re-fill the 2px orbital again
All this means that we cannot fill the orbitals with double electrons if the other orbitals in the same level are still empty, a minimum of 1 electron must fill each orbital in the same energy level first then it can re-fill them to reach the stable double electrons in opposite spins or directions, so the lewis dot diagram for oxygen would be as the following picture below
Single, Double & Triple Bonds
Single bond, is the bond in which the atoms are held together by only one electron pair, which means each atom contains one free electrone so both atoms will shair those electrons to form a bond like in chlorine atom
Double bond in which the atoms share 2 electron pairs like in sulphur monoxide
Triple bonds is the bond which the atoms share three electron pairs like in Nitrogen Molecule
So in general the number of bonds formed by an atom is related to its valence, the outershell unpaired electrons basically plays the major role in forming the different chemical bonds between different atoms
BOND STRENGTH & BOND LENGTH
Bond strength is the energy released by the formation os a molecular bond, for any bond to be formed (normally) an energy must be released when the electrons are shared & orbitals are semi-united
Bond Dissociation Energy is the energy needed to break a bond, I can explain it in this manner, when we formed a bond we needed to release energy to form this bond & when we want to break it, we need also to return this energy (give energy) for it to be broken, giving energy will activate and move the electrons to leave the octect shells & the pairing system more easier, the stronger the bond is, so the higher the energy needed to break it
This bond dissociation energy is holding the unit of Kcal\mol as the following examples
H-H Bonds needs 104 Kcal\mol energy to break
F-F Bonds needs 36 Kcal\mol energy to break
CH3-CH3 Bonds needs 83 Kcal\mol energy to break
CH2=CH2 Bonds needs 146 Kcal\mol energy to break
CH=-CH Bonds needs 200 Kcal\mol energy to break
F-F Bonds needs 36 Kcal\mol energy to break
CH3-CH3 Bonds needs 83 Kcal\mol energy to break
CH2=CH2 Bonds needs 146 Kcal\mol energy to break
CH=-CH Bonds needs 200 Kcal\mol energy to break
The Bond Length is the distance between the 2 nuclei in the molecular structure & it’s unit is ANGSTROM A degree as the following examples
H-H Have the distance of 0.72 A
CH3-CH3 Have the distance of 1.54 A
CH2=CH2 Have the distance of 1.34 A
CH=-CH Have the distance of 1.20 A
Note that the symbol =- means triple bond & from the past examples we recognize the following that by increasing the number of bonds we will need higher energy levels to break them, & by increasing the number of bonds the distance or bond length will decreaseCH3-CH3 Have the distance of 1.54 A
CH2=CH2 Have the distance of 1.34 A
CH=-CH Have the distance of 1.20 A











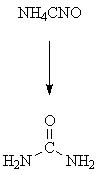















A large part of Alfa Chemistry's customers are pharmaceutical and biotechnology companies, including Pfizer, Novartis, Merck & Co., Johnson & Johnson, AstraZeneca, and Bayer. Alfa Chemistry is also a preferred partner for many universities and non-profit institutes.
ردحذفDOFL-NPB