Hydrocarbons are organic compounds that contain only 2 elements which are Carbon & Hydrogen atoms only & they are normally devided into 2 classes which are as the following
A) Aliphatic Hydrocarbons divided also into 3 subclasses which are Alkanes, Alkenes & Alkynes
B) Aromatic Hydrocarbons which are compounds containing Benzene structures
B) Aromatic Hydrocarbons which are compounds containing Benzene structures
Note that the alkanes are also called Saturated Hydrocarbons because they are stable regarding chemical reactivity but Alkenes & Alkynes are called Un-Saturated Hydrocarbons because they contain double & triple bonds which are active from chemical point of view, this will be explained in more details in the future articles, the following pictures will show general differences in basic structure between ETHANE (alkane), ETHENE (alkene) & ETHYNE (alkyne) regarding the bonds characteristics
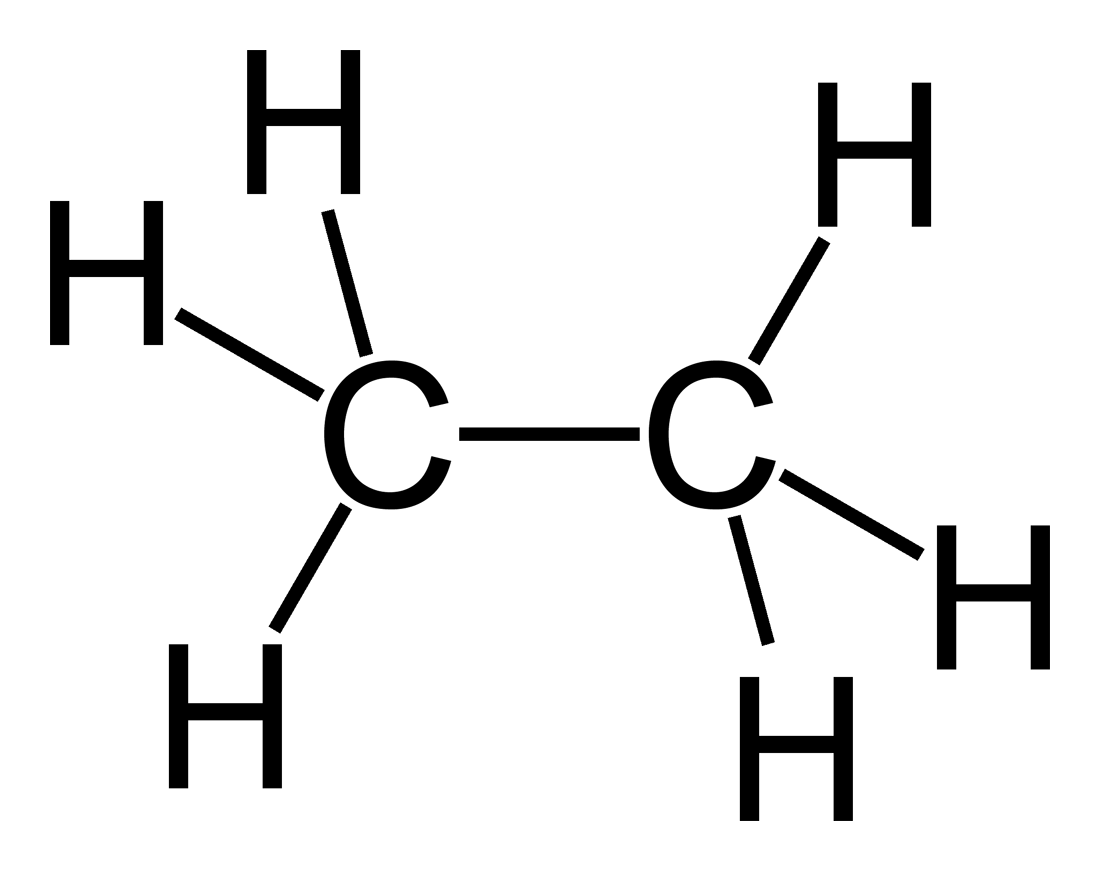
structure of ethane an alkane
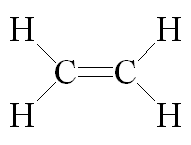
Structure of ethene an alkene
Structure of ethyne an alkyne
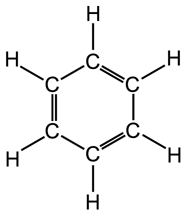
Structure of benzene ring in aromatic hydrocarbons
ALKANES
Alkanes are characterized by carbon-carbon single bond, that’s why they are reffered to as Saturated Hydrocarbons as just mentioned before, they show a general lack of chemical reactivity, therefore, they are also called Paraffin hydrocarbons, they are extremely flammable, they constitute the fuels for heating gases in our homes
STRUCTURE OF ALKANES
sp3 Hybridization
For a normal carbon atom in which z=6 the electronic configuration would be as the following please revise the article before this one in the same section
The first energy level K= 1s2
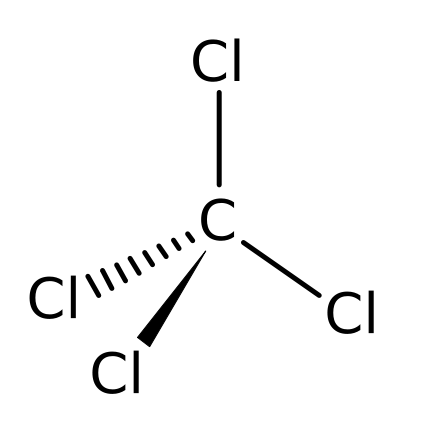
The 2′nd energy level L= 2s2, 2Px1, 2Py1 in this way we spent the 6 electrons 2 filled the first 1s orbital, 2 filled the 2′nd 2s orbital in L level & 2 left, one in the 2Px & 1 in the 2Py & the 2Pz will stay empty, this is the logical way of chemical distribution of the electrons (revise the first article on organic chemistry) & this means that carbon is suppose to do 2 bonds only (which means to be divalent) because it have only 2 un-shared free electrons, but in fact, carbon atoms showed to have 4 single bonds and to be tetravalent instead of being divalent with 2 single bonds only, for example as seen in ethane structure just mentioned upstairs, we see that a single carbon atome forms 4 single bonds or in the following structure of Carbon Tetrachloride, in which it formed 4 single bonds with 4 chloride atoms
The 2′nd energy level L= 2s2, 2Px1, 2Py1 in this way we spent the 6 electrons 2 filled the first 1s orbital, 2 filled the 2′nd 2s orbital in L level & 2 left, one in the 2Px & 1 in the 2Py & the 2Pz will stay empty, this is the logical way of chemical distribution of the electrons (revise the first article on organic chemistry) & this means that carbon is suppose to do 2 bonds only (which means to be divalent) because it have only 2 un-shared free electrons, but in fact, carbon atoms showed to have 4 single bonds and to be tetravalent instead of being divalent with 2 single bonds only, for example as seen in ethane structure just mentioned upstairs, we see that a single carbon atome forms 4 single bonds or in the following structure of Carbon Tetrachloride, in which it formed 4 single bonds with 4 chloride atoms
So to explain this, the theory of sp3 Hybridization was discovered in which by providing energy to the carbon atom, one electron from the ground state of 2s2 orbital will promote & be activated to leave the orbital & go to fill the empty 2Pz orbital in the L level state
So carbon will leave from the ground state to become in the activated state & then the 2s orbital will mix (hybridize) with the rest 2P orbitals to form 4 hybridized orbitals , for sure they have to hybridize to have 4 equal bonds, the process is shown in the following picture, note that if they didn’t hybridize this means that 4 single bonds will occur, 3 of them are equivalent (3 from the p orbitals) and one will be not equivalent to them because it merge from a lower energy level
So Hybridization is the merging of an s orbital & 3 p orbitals to give 4 equivalent sp3 orbitals, this hybridization will be by head-head of the orbitals forming what is known as sigma bond & giving the carbon a tetrahedron structure like that of methane the simplest organic compound in nature which is composed of only one carbon atom only
The structure of the first member in organic compounds & alkanes which is the simplest form composed of one carbon atom only & called methane & is not available purely directly in nature
Note that the new energy hybridization of the orbitals formed a new orbital called sp3 orbital which an energy which is higher then the 1s orbital & lower then the 2p orbitals
ETHANE
This is the 2′nd member of the alkane series, it’s composed of 2 carbon atoms so it’s molecule is larger then methane by -CH2- only & note that the difference between each member & the other in alkanes would be the same -CH2 molecule so we can say that methane is CH4 but ethane is C2H6 or CH3-CH3
PROPANE
It is the 3′rd member so it have one more carbone atom -CH2 compared to ethane & the structure would be C3H8 or CH3-CH2-CH3, as just mentioned, the fact is that every member of the alkanes series differs from the next member by a methylene group which is -CH2, the alkanes series is a homologous series
ISOMERIC STRUCTURE OF ALKANES
Isomers are different compounds with identical molecular formulas, they are readily distinguishable from each other because they exhibit differences in their physical & chemical properties, so they exhibit different chemical reactions
The first 3 members of the alkane series, the methane, ethane & propane have NO ISOMERS because there is only one way in arranging the molecule in 3D structures but the rest of the alkanes series might have an isomer, which is called structure isomers, see the following example
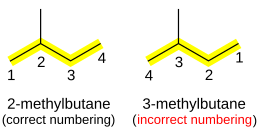
By comparing the structures of butane (the 4′th member in the series) & iso-butane we see that they have different molecular structure although they have the same number of carbon & hydrogen atoms, both have the same chemical formula which is C4H10 but they still differ in their properties & they are considered 2 different compounds (structural isomers) the first having a boiling point of zero degree C & the 2′nd having a boiling point of -12 degree C also from the past boiling points we can see that the straight chain are more stable than the isomer form, note that straight chains naming starts by the letter n, so we say n-butane & not butane alone to differentiate it from iso-butane
THE NOMENCALTURE OF ALKANES
The alkanes general formula is CnH(2n+2) which is stable for all the alkanes members, where n= the # of carbon atoms in the molecular structure
There are 2 basic systems for the naming of alkanes which are the following
A) Common Names , also called general or primary names or the un-systematic name
B) The IUPAC Name, or the systematic name
B) The IUPAC Name, or the systematic name
A)The Common Names of Alkanes
These are as the following
Holding 1 carbon, named METHANE, with a molecular formula of CH4 & Structural formula of CH4
Holding 2 carbons, named ETHANE, with a molecular formula of C2H6 & Structural formula of CH3-CH3
Holding 3 carbons, named PROPANE, with a molecular formula of C3H8 & Structural formula of CH3-CH2-CH3
Holding 4 carbons, named BUTANE, with a molecular formula of C4H10 & Structural formula of CH3-CH2-CH2-CH3
Holding 5 carbons, named PENTANE, with a molecular formula of C5H12 & Structural formula of CH3-CH2-CH2-CH2-CH3
Holding 6 carbons, named HEXANE, with a molecular formula of C6H14 & Structural formula of CH3-(CH2)4-CH3
Holding 7 carbons, named HEPTANE, with a molecular formula of C7H14 & Structural formula of CH3-(CH2)5-CH3
Holding 8 carbons, named OCTANE, with a molecular formula of C8H18 & Structural formula of CH3-(CH2)6-CH3
Holding 9 carbons, named NONANE, with a molecular formula of C9H20 & Structural formula of CH3-(CH2)7-CH3
Holding 10 carbons, named DECANE, with a molecular formula of C10H22 & Structural formula of CH3-(CH2)8-CH3
Holding 2 carbons, named ETHANE, with a molecular formula of C2H6 & Structural formula of CH3-CH3
Holding 3 carbons, named PROPANE, with a molecular formula of C3H8 & Structural formula of CH3-CH2-CH3
Holding 4 carbons, named BUTANE, with a molecular formula of C4H10 & Structural formula of CH3-CH2-CH2-CH3
Holding 5 carbons, named PENTANE, with a molecular formula of C5H12 & Structural formula of CH3-CH2-CH2-CH2-CH3
Holding 6 carbons, named HEXANE, with a molecular formula of C6H14 & Structural formula of CH3-(CH2)4-CH3
Holding 7 carbons, named HEPTANE, with a molecular formula of C7H14 & Structural formula of CH3-(CH2)5-CH3
Holding 8 carbons, named OCTANE, with a molecular formula of C8H18 & Structural formula of CH3-(CH2)6-CH3
Holding 9 carbons, named NONANE, with a molecular formula of C9H20 & Structural formula of CH3-(CH2)7-CH3
Holding 10 carbons, named DECANE, with a molecular formula of C10H22 & Structural formula of CH3-(CH2)8-CH3
Note that the names of all the members will end with -ane- which is derived from the family name Alkane
Complicated alkanes usually have relatively small substitutes called "Alkyl Groups" attached to the parent straight chain of carbon base, so in this case the alkane is named as an Alkyl Halide, the following picture is an example for an alkyl halid or a complicated alkane
The alkyl group is a hydrocarbon part of a mono-substituted alkane, the letter R is used to depict an alkyl group like the following structure
The general formula for alkyl goups is CnH(2n+1) differing than the formula of Alkanes itself by having one less Hydrogen in the structure, this is for sure because the alkyl group attached to the alkane will replace a hydrogen in the molecular structure, those alkyl groups are named by replacing the (-ane) part of the name of the parent alkane by (-yl) ending, so methane will be methyl & propane will be propyl & so on…..etc
B) The IUPAC Names of hydrocarbons
These are the systematic names of alkanes, by the way the word IUPAC refers to the following International Union of Pure and Applied Chemistry, the IUPAC system of nomencalture follows the following rules indicated below
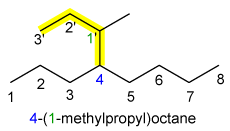
A) Select as the parent structure the longest continous chain of carbons
B) Number the parent carbon chain starting from whichever end will give the lowest number for the point of attachment of the substituent
C) If the same alkyl group (substituent) occurs more than once on the parent carbon chain the prefixes di, tri, tetra, penta …etc are used to indicate wether there are 2, 3, 4 or five or more substituents attached to the chain + giving numbers for different kinds of substituents
D) If several different substituents are attached on the parent carbon chain, they are named either in order of increasing complexity or in alphabetical order, see the following examples of naming
B) Number the parent carbon chain starting from whichever end will give the lowest number for the point of attachment of the substituent
C) If the same alkyl group (substituent) occurs more than once on the parent carbon chain the prefixes di, tri, tetra, penta …etc are used to indicate wether there are 2, 3, 4 or five or more substituents attached to the chain + giving numbers for different kinds of substituents
D) If several different substituents are attached on the parent carbon chain, they are named either in order of increasing complexity or in alphabetical order, see the following examples of naming
See the past picture & watch the naming process
A) We have to choose the parent chain, try counting all the carbons from left to right than from right to left then from down to left …etc you’ll find that the longest continous carbon chain would be composed of 5 carbons & by revising the table of alkane members we’ll find that the name would be methane, ethane, propane, butane & pentane, so the name of the parent chain would be pentane the 5′th member of the members holding 5 carbons
B) Now we have an attached one carbon molecule (alkyl group) because it is not included in the parent chain so because it is composed of one carbon atom we’ll call it methane & because it is an alkyl group so the name would turn to be methyl
C) Now we need to give numbers, if you try numbering the chain from all sides you’ll find 2 ways only, either the methyl group will be attached to the carbon # 4 or in other numbering way it will be attached to carbon number 2 & as the IUPAC rules insist to have the lowest number so we’ll choose number 2 carbon
D) So the name would be 2-methyl pentane as written in arabic in the picture
ANOTHER EXAMPLE
A) The parent chaain will be pentane also
B) The substitutes are methyl & ethyl groups attached to the same carbon atom
C) The lowest number would be 3
D) So the name would be 3-methyl-3-ethyl pentane (according to complexity) or 3-ethyl-3-methyl pentane according to alphabetical order of nameing the substitutes
B) The substitutes are methyl & ethyl groups attached to the same carbon atom
C) The lowest number would be 3
D) So the name would be 3-methyl-3-ethyl pentane (according to complexity) or 3-ethyl-3-methyl pentane according to alphabetical order of nameing the substitutes
ANOTHER EXAMPLES
CONFORMATION OF ALKANES
Structures of alkanes that differ from one another because of the rotation of one or more carbon atoms but that are identical as to the attachment of their atoms (not isomers) are said to be having different conformations, & each structure is reffered to as a conformer, they even have the same IUPAC name
Conformers are not isomers, because they are identical but they differ only by the rotation of one or more carbon atoms see the following examples of conformations of ethane
SAWHORSE CONFORMATION
NEWMAN PROJECTION
PERSPECTIVE REPRESENTATION
In the last perspective one note that the dotted line means behind (inside) the page in way, the normal line is exactly on the page & the last line is going toward you, toward outside the page or the screen
SOURCES OF ALKANES
The 2 principal sources of alkanes are Petroleum & Natural Gas, they constitute the chief sources of alkanes up to 40 carbons, as well as many aromatic, alicyclic (cyclic aliphatic hydrocarbons) & heterocyclic compounds which are cyclic molecules with more than one kind of atom in the ring other than carbon
The natural gas is consisted of low molecular weight alkanes from C1 up to C8 only because they are lighter & can be in natural gas form, by increasing the amount of carbons in the compounds the stability will increase & the boiling point will increase also and they will turn to become in a solid state
PHYSICAL PROPERTIES OF ALKANES
A) Ocuurence : at room temperature alkanes occur as gases, liquids & solids depending on the number of carbons available in the compounds, from C1-C4 are gases, From C5-C17 are liquids, From C18 & larger are solid waxes
B) Solubilities, Alkanes are considered to be a non-polar compounds, their solubility characteristic may be predicted by the law of like dissolves like which means that non-polar compounds are soluble in other non-polar compounds & polar compounds are generally soluble in other polar compounds
Thus alkanes are soluble in the non-polar solvents like carbon tetrachloride CCL4 & benzene C6H6 but they are insoluble in polar solvents such as water or alcohols
C) Boiling points, Generally the boiling point of n-hydrocarbons increases with increasing the molecular weight or the number of carbon atoms in the molecule, by each more carbon atom around 20-30 degree’s centigrate is increases as shown in the plot below
Straight chain alkanes have higher boiling points than their corresponding branched isomers which means that they are more stable also as just mentioned before
D) Melting Points, The melting points of alkanes also increases with the molecular weight just like the boiling points & there is no regularity regarding the differences in melting points between straight chain & branched chain compounds


















ليست هناك تعليقات:
إرسال تعليق