Phytochemistry, Terpenoids
Clinical Pharmacy, Phytochemistry Section
A study in Phytochemistry which is the chemistry of plants ingredients as a revision for professionals & students
A study in Phytochemistry which is the chemistry of plants ingredients as a revision for professionals & students
Studied & Revised By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
هذه مقالة تعنى بدراسة فصيلة كيميائية نباتية, تعتبر من أهم مكونات النبات الكيميائية وتسمى (التيربينويدز) وتدرسها من ناحية كيمياء النبات الطبية, وتعتبر التيربينويدز من مكونات النبات العضوية الأساسية كيميائيا, ولها استخدامات مهمة في مجالي الطب والصيدلة وحتى الصناعات البيولوجية, وهي من المواد الدهنية التكوين, والتي تتواجد عادة في سيتوبلازم الخلية النباتية الحية, وتعتبر العناصر الصغيرة في حجمها الكيميائي (كمية الكربون المحتواة) من العناصر الطيارة ذات الروائح المميزة كمثل تلك التي تعطي النعناع رائحته او الكافور وما الى ذلك, ولها تطبيقات متعلقة بالحشرات أيضا والانسان
دراسة ومراجعة اسماعيل المرتضى
يمكن التزويد بالمراجع المستخدمة عند الطلب
Terpenoids are the largest & structurally the most various class of naturally occuring secondary plant metabolites, They are found normally in all plants (higher & lower) + in animals + in insects, They are generally lipid soluble & are located in the cytoplasm of the plant cell, Most of them occur free in the plant tissue but many are found as glycosides & esters of organic acids & in some cases in combination with proteins, some organic acids are lipids + non-polar organic solvents which are soluble with cinnamic or acetic acid which are the most common
Terpenoids are the most diverse chemical group naturally, some are acyclic & the others are cyclic
Extraction & Detection
Terpenoids are normally extracted from plant tissues with petroleum ether, chloroform & diethyl ether. They can be separated chromatographically on silica gel or alumina, The lower members are volatile so that they can be obtained by distillation methods
All the terpenoids can be extracted by non-polar solvents
They are colourless (except carotenoids) & there is no sensitive specific chromato-reagent for their detection, the common reagents used for their detection are vanillin\H2SO4, antimony trichloride\HCL & anisaldehyde\H2SO4
TERPENOIDS FUNCTIONS
A number of quite different functions had been described such as the following
A) As growth regulators; sesqui-terpenoids (abscisins) are growth inhibitors while di-terpenoids (gibberellins) are promotors
B) The coloured terpenoids (like carotenoids) are involved as an accessary pigments in the process of photosynthesis
C) Some lower terpenoids provide plants with distinct smells (as I said before, the lower members are volatile in nature like most of the chemical compounds of low organic formula) so acting as insect attractants or repellant, used for defence
D) Some non-volatile terpenoids have been implicated as sex hormones among fungi, while others act as agents for communication among insects like ants
A) As growth regulators; sesqui-terpenoids (abscisins) are growth inhibitors while di-terpenoids (gibberellins) are promotors
B) The coloured terpenoids (like carotenoids) are involved as an accessary pigments in the process of photosynthesis
C) Some lower terpenoids provide plants with distinct smells (as I said before, the lower members are volatile in nature like most of the chemical compounds of low organic formula) so acting as insect attractants or repellant, used for defence
D) Some non-volatile terpenoids have been implicated as sex hormones among fungi, while others act as agents for communication among insects like ants
So we can see the huge diversity in the functions of this important natural chemical class in the biology of plants & animals in general
TERPENOIDS CHEMISTRY
Terpenoids are related by a common origin & a common structural relationship,so although they have a wide diversity but still they are classed under the same origin chemically, They are composed of multiples of a five carbon atom unit (or its isomer) known as ISOPRENE (iso-pentenyl unit, prenyl, C5H8) for this, they are often referred as isoprenoid compounds, the unit is shown in the following pictures
The unit is a C5H8 unit & is composed of a figure called (Head) which contains a =CH2 & a methylene group (CH3) & a (TAIL) composed of only =CH2 & a normal hydrogen, resonance may occur between the carbon atoms also due to the appearance of a 2 double bonds in the isoprene structure
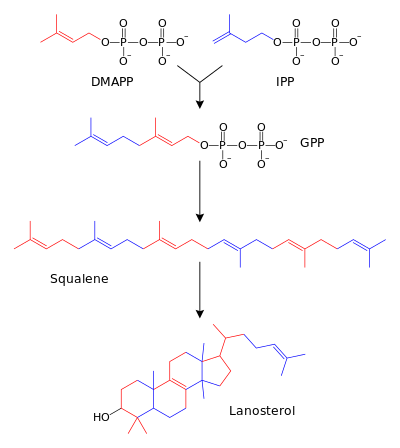
This simple 5 carbon unit is the responsible for the key reactions in terpenoidal biosynthesis, The union of two C-5 residues in a head to tail manner with the formation of a C-C carbon gives rise to the C-10 unit (geranyl pyrophosphate), Extension of this unit by the addition of a third unit gives (farnesyl pyrophosphate) which is C-15 & continuation of the process yield C-20, C-25, C-30, C-40…etc and the process can continue to give polyprenyl groups
This simple 5 carbon unit is the responsible for the key reactions in terpenoidal biosynthesis, The union of two C-5 residues in a head to tail manner with the formation of a C-C carbon gives rise to the C-10 unit (geranyl pyrophosphate), Extension of this unit by the addition of a third unit gives (farnesyl pyrophosphate) which is C-15 & continuation of the process yield C-20, C-25, C-30, C-40…etc and the process can continue to give polyprenyl groups
We need to note that sometimes some compounds might be included also in the building process which are not exactly isoprene units although they have similar shape and emperical formula, but not identical, they also might have a head & tail phenomena like the dimethylallyl units
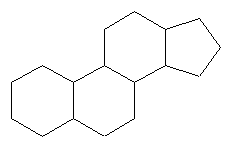
The building unit Isoprene does not occur as free in nature but it occurs as a pyrophosphate units, so it’s called Isoprenyl pyrophosphate units IPPP or Dimethylallyl pyrophosphate DMPP or one of their derivatives & both of those units are the actual units responsible for the building of terpenoids & their biosynthesis in general as just mentioned before, so a revision for the multiplication process we see that for example the 2′nd unit formed would be composed of c-10, linked in a head to tail manner with some exceptions some times, the head to tail manner of linkage is called the Isoprene Rule & at the end if the length increases than the tendency to be cycled (cyclization) will also increase naturally
Note also that when 2 simple C-5 pyrophosphate units are united, the reaction will yield for example one simple C-10 geranyl pyrophosphate & the resulted structure will contain only one pyrophosphate unit inside it, that means that the reaction will cleave one pyrophosphate from one of the entered units and free it away
Classification of Terpenoids
They are classified according to the number of isoprene units involved in their formation into the following
A) Hemi-terpenoids (or Hemiterpenes), C5H8, 1 unit
B) Mono-terpenoids, C10H16, 2 units
C) Sesqui-terpenoids, C15H24, 3 units
D) Di-terpenoids, C20H32, 4 units
E) Ses-terpenoids, C25H40, 5 units
F) Tri-terpenoids, C30H48, 6 units
G) Tetra-terpenoids (carotenoids), C40H64, 8 units
H) Poly-terpenoids, (C5H8)n, which is > 8 units
A) Hemi-terpenoids (or Hemiterpenes), C5H8, 1 unit
B) Mono-terpenoids, C10H16, 2 units
C) Sesqui-terpenoids, C15H24, 3 units
D) Di-terpenoids, C20H32, 4 units
E) Ses-terpenoids, C25H40, 5 units
F) Tri-terpenoids, C30H48, 6 units
G) Tetra-terpenoids (carotenoids), C40H64, 8 units
H) Poly-terpenoids, (C5H8)n, which is > 8 units
Note that the first one which is CH8, the Hemiterpenoid, doesn’t occur naturally in nature alone & also note that the # of additional carbon atoms will not change, but still the hydrogen amount is not considered to be added in a stable manners & this is a very important point so that not to have a fast guess without studying the structure itself, so there is no direct imperical formula or relation between the # of carbon atoms & the relative number of the hydrogen atoms, unlike normally most of the basic organic compounds
HEMI-TERPENOIDS, C5H8
Compounds of this class as mentioned several times before are rarely encountered in nature as stable, isolable metabolic end products, but they exist in living cells as highly active substances, for example dimethylallyl pyrophosphate (DMPP) and Isopentenyl pyrophosphate IPPP, the difference between both of them is the location of the 2 double bonds within the internal structure of the simple isoprene unit itself
Note that dimethylallyl pyrophosphate might be also abreviated as DMAPP
See the following picture of a saturated resonance IPPP
See the following picture of a saturated resonance IPPP
The pysophosphate units are the responsible for forming the linkage between 2 isoprene units in general
IPPP & DMPP are the key compounds used for the biosynthesis of terpenoidal compounds, they are considered as the Building Blocks for Terpenoids in chemistry terms
IPPP & DMPP are the key compounds used for the biosynthesis of terpenoidal compounds, they are considered as the Building Blocks for Terpenoids in chemistry terms
MONO-TERPENOIDS, C10H16
They are derived from GPP (geranyl pyrophosphate) by different reactions (oxidation or dehydration) that do not change the number of the carbon atoms, they appear free in nature or combined with sugars & proteins & are classified into 4 major categories as the following
A) Open chain monoterpenoids, acyclic
B)
المزيدA) Open chain monoterpenoids, acyclic
B)
Phytochemistry, Lipids
مايو 6th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Phytochemistry,Phytochemistry, Lipids
Clinical Pharmacy, Phytochemistry Section
A study in Phytochemistry which is the chemistry of plants ingredients as a revision for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
هذه مقالة سريعة جدا تعنى بدراسة التكوين الكيميائي للدهون النباتية, وهي غير شاملة ولكنها مقتطف سريع جدا حيث ان هذه المقالة تتبع قسم (كيمياء النبات) والذي لا يفصل كثيرا في بعض الأمور, وسادرج مقالات أخرى أكثر توسعا حول الموضوع عندما نتناوله من جانب الكيمياء العضوية حيث أنه يكون متشعبا أكثر وأكثر أهمية, اما كيمياء النبات فهي تعنى بأمور تخصصية معينة في تركيبة المواد الكيمياء الطبيعية المختلفة
دراسة ومراجعة اسماعيل المرتض
يمكن التزويد بالمراجع المستخدمة عند الطلب
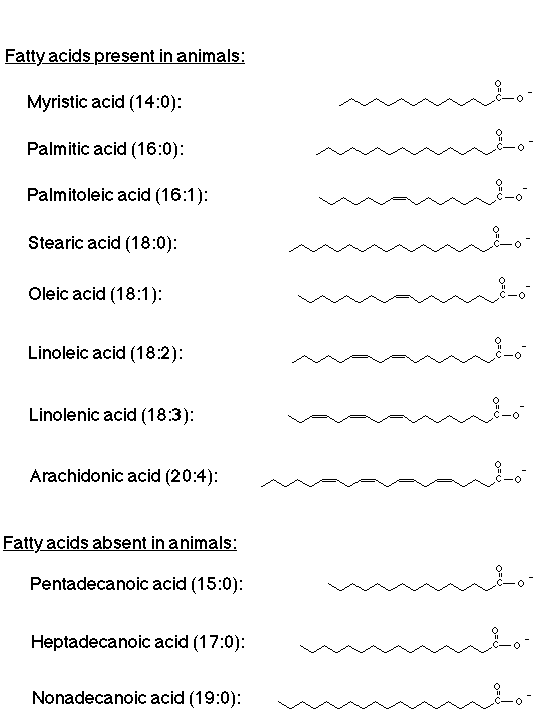
Lipids are substances of biological origin that dissolve in many organic non-polar solvents, But are insoluble or very slightly soluble in water. Lipids are usually compounds of Fatty Acids or complex alcohols esters or even having different structure. Their major function s to store food energy, Fatty acids are mono-carboxylic Acids, usually having 21 to 22 carbons with one or more double bonds, Lipids generally includes the following
A) Simple Lipids, such as Fixed oils, Fats & Waxes
B) Complex Lipids such as phophotides & Lecithins which contain phosphorous & nitrogen in addition to carbon, hydrogen & oxygen
B) Complex Lipids such as phophotides & Lecithins which contain phosphorous & nitrogen in addition to carbon, hydrogen & oxygen
FIXED OILS, FATS & WAXES
Fixed oils & fats are esters of long chain fatty acids with a tri-hydric alcohol (glycerol), Usually the glycerides of un-saturated fatty acids are liquid. whereas the glycerides of saturated fatty acids of sufficient chain length are solids, check the following pictures to have a more clear understanding about the structure of fatty acids & glycerides esters
Fixed oils & fats are naturally occuring mixtures of lipids widely distributed in both animals & plants. The word "Fixed Oils" & "Fats" are interchangable according to the lipid being solid or liquid at room temperature
Most lipids from vegetable origin are liquids (cottonseed oil) with few exceptions like (theobroma oil = cocoa butter), While lipids from animal origin are semi-solids or solids (like lard) with the exception of cod liver oil
Oils & Fats of vegetable origin are obtained by expression or by solvent extraction, If expression is carried out in the cold, the oil is known as "Virgin Oil" or a "Cold Pressed Oil", In contrast; if the process is carried out in heat, the oil is known as "Hot-Pressed Oil", Animal fats are separated from other tissues by rendering with steam, with or without pressure. The heat melts the fats, which rises to the top to be separated by decantation
Fixed oils are classified into drying, semi-drying & non-drying, a classification based on their ability to absorb oxygen from the air
There are several pharmacopeial tests based upon chemical constitution of fatty acids, These tests are used to determine the identity, quality & purity of fixed oils, USP recommends the acid value (acid number ), saponification value, iodine number & Reichert-Meissl number
A) The Acid Value: It is a number of mg of KOH required to neutralize the free fatty acids in 1g of the substance, for example the acid value of Castor Oil must not exceed 2
B) The Saponification Number: It is the number of mg KOH required to neutralize the free fatty acids resulting from the complete hydrolysis of 1g of the substance
C) The Iodine Number: It is the number of g of Iodine, absorbed by, under prescribed conditions, by 100g of the substance
D) Reichert-Leissl Number: It is the number of ml. of 0.1N KOH required to neutralize the volatile water soluble acids obtained by the hydrolysis of 5g of the fat
B) The Saponification Number: It is the number of mg KOH required to neutralize the free fatty acids resulting from the complete hydrolysis of 1g of the substance
C) The Iodine Number: It is the number of g of Iodine, absorbed by, under prescribed conditions, by 100g of the substance
D) Reichert-Leissl Number: It is the number of ml. of 0.1N KOH required to neutralize the volatile water soluble acids obtained by the hydrolysis of 5g of the fat
Waxes are esters of fatty acids & large mono-hydroxy alcohols (chain or cyclic) of high molecular weight such as for example cetyl alcohol
المزيدPhystochemistry, Carbohydartes
أبريل 16th, 2009 كتبها اسماعيل العبد مرتضى نشر في , Pharmaceutical Biochemistry, Phytochemistry,Phytochemistry, Carbohydartes
Clinical Pharmacy, Phytochemistry Section
A study in Phytochemistry which is the chemistry of plants ingredients as a revision for professionals & students
Done By Ismail Mortada
B.Sc.Pharmacy & Health Sciences, Clinical Pharmacy Section
Carbohydrates can be defined as an aldehyde or ketone alcohols containing carbon, hydrogen & oxygen, in which hydrogen & oxygen are generally the same ration as in water, This definition is not absolutely true because there are compounds that conform the hydrate rule but are not true carbihydrates like formaldehyde (H.CHO), There are also some compounds that have the chemical properties of carbohydrates but do not donform to the hydrate rule such as the deoxy sugars like rhamnose, C6H12O5
CLASSIFICATION
According to the complexity of nature, carbohydrtaes are classified into the simple sugars (saccharides) & the polysaccharide complex sugars, between them we might have disaccharides also
A) SIMPLE SUGARS PR SACCHARIDES
According to the number of sugars, they are classified into the following
A) Monosaccharides
B) DIsaccharides
C) Trisaccharides
D) Tetrasaccharides
E) Oligosaccharides
B) DIsaccharides
C) Trisaccharides
D) Tetrasaccharides
E) Oligosaccharides
A) MONOSACCHARIDES
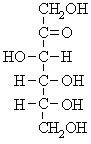
These are compounds containing only one sugar unit or saccharide & cannot be hydolyzed to a more simpler form, According to the number of oxygen or carbon atoms, simple sugars are subclassified into bioses, trioses, tetroses, pentoses & hexoses. They further can be subdivided into aldoses & ketoses depending upon wether these sugars contains an aldehyde or ketone group, respectively, Hexoses often exist in cyclic forms as well as straight chain structures, The cyclic forms are either with 6-membered-pyranose ring or with 5-membered-pyranose ring as shown in the following pictures of D-Glucose an aldohexose & D-Fructose an ketohexose
These are compounds containing only one sugar unit or saccharide & cannot be hydolyzed to a more simpler form, According to the number of oxygen or carbon atoms, simple sugars are subclassified into bioses, trioses, tetroses, pentoses & hexoses. They further can be subdivided into aldoses & ketoses depending upon wether these sugars contains an aldehyde or ketone group, respectively, Hexoses often exist in cyclic forms as well as straight chain structures, The cyclic forms are either with 6-membered-pyranose ring or with 5-membered-pyranose ring as shown in the following pictures of D-Glucose an aldohexose & D-Fructose an ketohexose
D-Glucose an aldohexose
D-Fructose a ketohexose
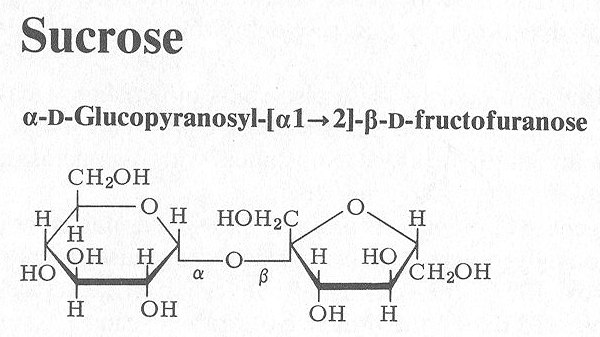
B) DISACCHARIDES
Upon hydrolysis, thise yield 2 monosaccharide molecules for example such as sucrose & lactose
Upon hydrolysis, thise yield 2 monosaccharide molecules for example such as sucrose & lactose
Sucrose is a combination of both (glucose + fructose), it is present abundantly in cane sugar & beet sugar, it is normally used in syrups as a demulcent & nutrient
Lactose is a combination of both (glucose + galactose), It is obtained from milk, it is less sweet than sucrose, it is used as a nutrient in infants food & as an inert diluent for tablets & other drugs
C) TRISACCHARIDES
For example raffinose which is a combination of (glucose + fructose + galactose), it occurs in small quantities in the beet sugar & in appreciable quantities in the cottonseed, soybean & other oil seeds
For example raffinose which is a combination of (glucose + fructose + galactose), it occurs in small quantities in the beet sugar & in appreciable quantities in the cottonseed, soybean & other oil seeds
D) TETRASACCHARIDES
For example is stachyose which is a combination of (glucose + fructose + galactose + galactose), it is the only known tetrasaccharide & is usually associated with sucrose & raffinose
For example is stachyose which is a combination of (glucose + fructose + galactose + galactose), it is the only known tetrasaccharide & is usually associated with sucrose & raffinose
E) OLIGOSACCHARIDE
They are considered as condensation products of up to 10 molecules of simple sugars
They are considered as condensation products of up to 10 molecules of simple sugars
THE POLYSACCHARIDES
These are compounds that yield a large & indefinite number of monosaccharides (heterogenous) on hydrolysis & are difficult to classify
According to the products of hydrolysis, they are classified into pentosans (those yielding pentoses among hydrolysis) & hexosans (those yielding hexoses among hydrolysis), Some polysaccharides complexes yields in addition to monosaccharides, their sulphate esters, uronic acids or amino-sugars, these polysaccharides are sometimes called Derived Carbohydrates as for example the following
A) Plant fibers
B) Starches
C) Peptic substabces
D) Seaweeds
E) Gums & Mucilages
B) Starches
C) Peptic substabces
D) Seaweeds
E) Gums & Mucilages
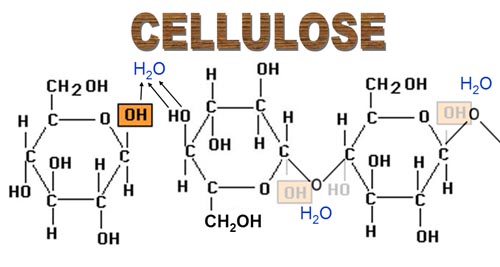
A) PLANT FIBERS
An example is cotton that is composed of 91% cellulose, cellulose is a linear polysaccharide consisting of 1,4-linked B-D-glucopyranose units
An example is cotton that is composed of 91% cellulose, cellulose is a linear polysaccharide consisting of 1,4-linked B-D-glucopyranose units
Cellulose —-> gives B-D-glucose, this reaction (among hydrolysis by conc.mineral acids at low temperature)as an important factor
Cellulose is the most widely distributed polysaccharide
B) STARCHES
They are widely distributed in plants, Starch is a mixture of structurally different 2 polysaccharides, these are Beta-amylose & amylopectin, Examples of plants containing starch are wheat, rice, potato & maize, Starch is used as a dusting powder, skin emollient & an an antidote in iodine poisoning, it has too many commercial uses
They are widely distributed in plants, Starch is a mixture of structurally different 2 polysaccharides, these are Beta-amylose & amylopectin, Examples of plants containing starch are wheat, rice, potato & maize, Starch is used as a dusting powder, skin emollient & an an antidote in iodine poisoning, it has too many commercial uses
C) PECTIN SUBSTANCES
These are colloidal carbohydrates of high molecular weight & rather complex composition, for example pectin which is present in the inner portion of the rind of citrus fruits, Pectin is used as a protectant & suspending agent & is an ingredient in many anti-diarrhoeal drugs
These are colloidal carbohydrates of high molecular weight & rather complex composition, for example pectin which is present in the inner portion of the rind of citrus fruits, Pectin is used as a protectant & suspending agent & is an ingredient in many anti-diarrhoeal drugs
D) SEAWEEDS
Examples are agar, alginic acid & Irish moss, Alginic acid is used as an emulsifying, stabilizing, gelling & thickening agent, Irish moss is used as a binder & emulsifying agent & in toothpastes
Examples are agar, alginic acid & Irish moss, Alginic acid is used as an emulsifying, stabilizing, gelling & thickening agent, Irish moss is used as a binder & emulsifying agent & in toothpastes
E) GUMS & MUCILAGES
Plantg gums are complex carbohydrates formed from sugar units joined to uronic acid resdues by glycosidic linkage forming branched chain structures
Plantg gums are complex carbohydrates formed from sugar units joined to uronic acid resdues by glycosidic linkage forming branched chain structures
These sugars may include methyl pentose residues like rhamnose or pentoses like D-xylose & L-arabinose or hexoses like D-mannose & D-galactose
RHAMNOSE D & L FORMS
D-XYLOSE
D-arabinose
D-mannose
D-galactose
The uronic acid residues are D-glucuronic acid & in some cases D-galacturonic acids as in tragacanth gum
D-galacturonic acid
D-glucuronic acid
Gums do not ferment by yeast, & they are soluble in water forming acidic solutions which is levorotatory, They are insoluble in alcohol, they have diverse application in pharmacy, they are ingredients in dental & other adhesives & in bulk forming laxatives
They are also used as tablet binders, emulsifiers, gelatinizing agents, suspending agents, stabilizers & thickeners
ACACIA GUM OR GUM ARABIC
It is one of the most important polysaccharide gums, obtained by icisions from trunk & branches of acacia senegal from the family, Leguminosae
It consists mainly of arabin, oxidase enzme & water, Arabin is the calcium salt of arabic acid with traces of magnseium & potassium salts, Arabic acid consists of 4-galactose + 2L-Arabinose + L-rhamnose + glucuronic acid, Hydrolytic products depend upon experimental conditions
Gum arabic is mainly used as a suspending agent in tablet granulation, as a demulcent & also in confectionery
HONEY
It is the sweet syrupy secretion deposited in the honeycomb of bee, Apis mellifera, family Apidae
Honey is usually onbtained by centrifugation followed by melting the syrupy product at a temperature less than 80 degree C, allowed to stand & impurities are skimmed off, Honey obtained by expression has high content of waxes, when it is frshly prepared, it is a clear syrupy liquid of a pale yellow or reddish brown colour, on keeping, it tends to crystallize & becomes opaque & granular, the colour and taste depend very largely on the flowers used for its preparation
Honey is mainly composed of invert sugar (euimolecular quantities of D-glucose & D-fructose) & water, It also contains small quantities of sucrose, dextrin, formic acid, volatile oil, wax, pollen grains, vitamins & minerals
Honey is used as a demulcent, nutrient & as a sweeting agent in food & pharmaceuticals

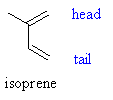




























ليست هناك تعليقات:
إرسال تعليق