| Introduction | ||||||
| Antibodies (Ab) are proteins produced by B lymphocytes of the immune system in response to foreign proteins, antigens (Ag). Abs function as markers, binding to the Ags, so that the Ag molecules can be recognised and destroyed by phagocytes. The part of the Ag that the Ab binds to is the epitope. The epitope is thus a short amino acid sequence that the Ab is able to recognise. Two features of the Ab epitope relationship are key to the use of monoclonal antibodies (mAb) as a molecular tool; (a) specificity: the Ab only binds to its particular epitope, (b) sufficiency: the epitope can bind to the Ab on its own, i.e. the presence of the whole Ag molecule is not necessary (Fig. 1). | ||||||
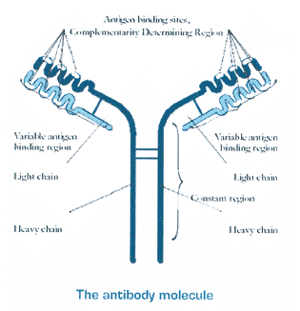 Fig. 1: The antibody molecule. | ||||||
| MAbs are Abs cultured from a population of B cells derived from a single, ancestral B cell. So, what are the applications of mAbs, pros and cons, and what have their uses brought to immunodiagnostics. | ||||||
| Basic background about mAbs | ||||||
| In the 1970’s Kohler and Milstein utilised somatic cell hybridization to develop and approach to immortalise individual Ab producing cells. As a result there is now the capability of generating cell lines that would produce a single Ab species (a mAb) (Fig. 2). A standardized procedure could be adapted and it would provide an ultimate source of the mAb, eliminating the need for highly purified Ags or immunisation, and allowing the investigator to design immunisation and screening strategies to generate highly specific monoclonal reagents towards | ||||||
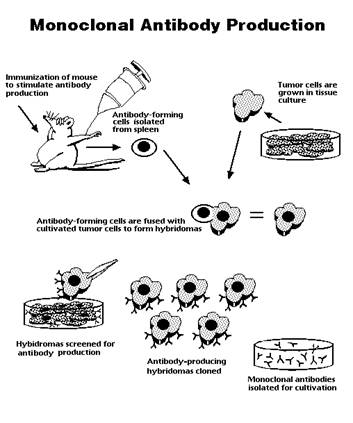 | ||||||
| Ags of scientific interest, (1). Monoclonal antibodies have been employed in investigative research to isolate, identify, and characterise molecular and cellular components of complex biological systems. In addition, mAbs are increasingly being utilized as highly specific reagents in the diagnosis of diseases and are being tested for their effectiveness as biological response modifiers, (4). Furthermore, mAbs have been incorporated into numerous strategies to identify biological and/or chemical substances in tissue or environmental samples. The ability of Abs to catalyse chemical reactions has recently been demonstrated suggesting that Abs capable of performing enzymatic functions could be designed, (3). | ||||||
| Applications of mAbs | ||||||
| MAbs were soon in routine use as well as research in over 100 laboratory techniques and had a profound impact on diagnostics, but immediate therapeutic uses were limited. So, perhaps the best example of the applications of mAbs is the generation of a large panel of reagents that define cell surface and make up our protective immune system. MAbs that define CD molecules and flow cytometry have been used to identify the normal components of the immune system and to determine if these components are under-represented or over produced. Moreover, mAbs reactive with the CD4 molecules expressed on helper cells are used to demonstrate that a decrease in CD4 cells is a feature of AIDS and that the levels can be used to stage the disease. Thus, mAbs have proven critical not only in allowing for an understanding of the complexity of the immune system but also in the understanding, diagnosis and management of the disease. What is more, mAbs have been extensively used in the design of sensitive detecti on assays such as ELISAs (e.g. viruses detection kits where different methods were used such as the sandwich, competition or direct techniques) used to detect normal and autoantibody levels (e.g. TSH), determining the presence and levels of auto antigens, viral/bacterial and other environmental Ags as well as assess the level of normal components in body fluids (Fig. 3 and Fig. 4). Another area that monoclonal antibodies have an impact on is the isolation and purification of molecules, (6, 1). | ||||||
| The field of molecular genomics has benefited from the availability of monoclonal antibody technology. For example, a known mAb that recognises a molecule of interest can be used to identify its gene. Also, mAbs have been included in many protocols as biological response modifiers. Abs against bioactive cytokines have been used in therapy for many immunologically based diseases such as the control of transplantation rejection (e.g. OK T3, an antibody on the T3 Ag of T cells) and the modulation of autoimmune diseases, (1). In addition, mAbs have also been used in applied chemistry. It was appreciated that enzymes function, in part, by having a high affinity interaction with a short-lived transition state. It was reasoned that, if enzymes can interact with these transitional states, maybe an Ab can as well and potentially serve as an enzyme. By generating Ab reagents against enzyme inhibitors that mimic the transitional state one can isolate Abs that can provide catalytic functions. The cata lytic reactions range from redox reactions to structural rearrangements. This initial success, together with the principles emerging in the field of combinatorial chemistry indicates that recognition molecules generated by the immune system have tremendous potential to be used as chemical tools, (2). | ||||||
| So, in short the applications of mAbs can be summarised into being: grouping blood types and identifying viruses, those mAbs that are tagged with fluorescent or radioactive labels and used in pregnancy, and heart disease, purification of drugs used in some therapies, and purging kidney transplants of donor lymphocytes to counteract rejection, (3). | ||||||
Fig. 3: Sandwich assay. | ||||||
Fig. 4: Competitive assay. | ||||||
| Advantages and disadvantages of mAbs | ||||||
| MAbs have many pros and cons, especially when it comes to comparing them with polyclonal antibodies. Since the isolation of mAbs, the use of polyclonal antibodies was limited, although these antibodies still have some important uses up till now. For example, polyclonal antibodies are less expensive to produce compared to mAbs which cost a lot to produce and are time consuming, (2). Also polyclonal antibodies often recognise multiple epitopes, making them more tolerant of small changes in the nature of the Ag and are the preferred choice for detection of denatured proteins. Moreover, polyclonal antibodies may be generated in a variety of species, including rabbit, goat, sheep, donkey, chicken and others, giving the users many options in experimental designs. On the other hand, one of the many disadvantages of polyclonal antibodies would be that the tests where they are used must be calibrated, or else, results will vary and maybe lost, (5). However, mAbs have many advantages, they are specific and thus are excellent as the primary Ab in an assay, or in detecting Ags in tissue, and will often give significantly less background staining than polyclonal antibodies. When compared to polyclonal antibodies, the homogeneity of mAbs is very high and they have an unlimited supply with defined characteristics. If experimental conditions are kept constant, results from mAbs will be highly reproducible, between experiments. What is more, specificity of mAbs makes them extremely efficient for binding an Ag within a mixture of related molecules, such as in the case of affinity purification. In addition, there are so many advantages of the uses of mAbs rather than polyclonal Abs in blood transfusion. For example, mAbs are extremely avid reagents (usually suitable for slide reactions), they do not require long incubation times nor the need for diluent controls and as a specific example in blood transfusion, patients with positive direct antiglobulin (mAb) test can be directly typed. But, mAbs do have some disadvantages, like; affinity, as the average affinity of mAbs is generally lower than polyclonal antibodies. Also, because the Ab is monoclonal it may not produce the desired biological response. Moreover, mAbs may be too specific or they may even cross react with some unrelated Ags or even with some naturally occurring Abs in some people’s blood, (1). What is more, a problem came to existence when mouse mAbs were used in therapy, as it turned out to be that mouse mAbs are of limited therapeutic use in humans because they are rapidly inactivated by a human immune response, preventing them from providing long-term therapeutic benefits, (3). This immune response can also cause flu-like symptoms, allergic reactions and in extreme cases, systemic shock, or death. Furthermore, mouse mAbs cannot efficiently activate other important human immune-system components. So, the need to make human mAbs was important for further advances in technology. One approach has been to engineer mouse/human hybrid Abs that still bind to Ags, but these seem to be less likely to provoke an immune response (Fig. 5). So, this was done by cloning mouse CDR into human framework region genes, (2). | ||||||
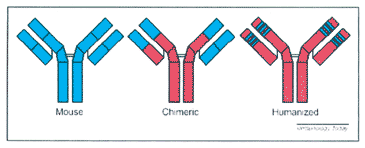 Fig. 5: Different Antibody Molecules. | ||||||
| These human mAbs seem to work and are thought to be superior to their predecessors. This can be done by another couple of methods, by producing them by phage or yeast display or by generating them in human immunoglobulin transgenic mice, (7). | ||||||
| Conclusion | ||||||
| In summary, we think in a few years’ time, mAbs would have a tremendous impact on the biological and chemical sciences. Their availability has allowed researches to ask new questions and to develop new insights and applications that would ultimately benefit the human health and condition. While new technologies are being developed that may eventually change how one proceeds to generate monoclonal reagents, it is clear that mAbs will continue to have an important and positive impact on scientific endeavours in the near future. | ||||||
| References | ||||||
| 1- Brich JR, Lennox ES. Monoclonal Antibodies: Principles and applications, 1995 2- McMichael A, Fabre J. Monoclonal antibodies in clinical medicine, 1982 3- Newell D, Mcbride B, Clark S. making monoclonals, 1989 4- Butt W. practical immunoassay: the state of the art, 1990 5- www.bbc.co.uk 6- www.chemicon.com/resource/ANT 101/al .asp 7- www.serologicals .com/products/blood typing.shtml | ||||||
الاثنين، 8 فبراير 2010
Monoclonal Antibodies
الاشتراك في:
تعليقات الرسالة (Atom)

ليست هناك تعليقات:
إرسال تعليق